Translocation and endocytosis for cell-penetrating peptide internalization
- PMID: 19833724
- PMCID: PMC2797166
- DOI: 10.1074/jbc.M109.056309
Translocation and endocytosis for cell-penetrating peptide internalization
Abstract
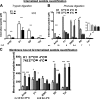
Cell-penetrating peptides (CPPs) share the property of cellular internalization. The question of how these peptides reach the cytoplasm of cells is still widely debated. Herein, we have used a mass spectrometry-based method that enables quantification of internalized and membrane-bound peptides. Internalization of the most used CPP was studied at 37 degrees C (endocytosis and translocation) and 4 degrees C (translocation) in wild type and proteoglycan-deficient Chinese hamster ovary cells. Both translocation and endocytosis are internalization pathways used by CPP. The choice of one pathway versus the other depends on the peptide sequence (not the number of positive changes), the extracellular peptide concentration, and the membrane components. There is no relationship between the high affinity of these peptides for the cell membrane and their internalization efficacy. Translocation occurs at low extracellular peptide concentration, whereas endocytosis, a saturable and cooperative phenomenon, is activated at higher concentrations. Translocation operates in a narrow time window, which implies a specific lipid/peptide co-import in cells.
Figures


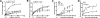
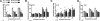


References
-
- Derossi D., Joliot A. H., Chassaing G., Prochiantz A. (1994) J. Biol. Chem. 269, 10444–10450 - PubMed
-
- Duchardt F., Fotin-Mleczek M., Schwarz H., Fischer R., Brock R. (2007) Traffic 8, 848–866 - PubMed
-
- Richard J. P., Melikov K., Vives E., Ramos C., Verbeure B., Gait M. J., Chernomordik L. V., Lebleu B. (2003) J. Biol. Chem. 278, 585–590 - PubMed
-
- Vivès E., Schmidt J., Pèlegrin A. (2008) Biochim Biophys Acta 1786, 126–138 - PubMed
-
- Console S., Marty C., García-Echeverría C., Schwendener R., Ballmer-Hofer K. (2003) J. Biol. Chem. 278, 35109–35114 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

