Leukocyte Ig-like receptor B4 (LILRB4) is a potent inhibitor of FcgammaRI-mediated monocyte activation via dephosphorylation of multiple kinases
- PMID: 19833736
- PMCID: PMC2787346
- DOI: 10.1074/jbc.M109.035683
Leukocyte Ig-like receptor B4 (LILRB4) is a potent inhibitor of FcgammaRI-mediated monocyte activation via dephosphorylation of multiple kinases
Abstract
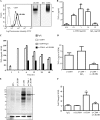
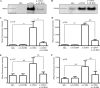
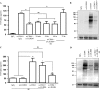
The leukocyte immunoglobulin-like receptor (LILR) B4 belongs to a family of cell surface receptors that possesses cytoplasmic immunoreceptor tyrosine-based inhibitory motifs (ITIMs). LILRB4 is believed to down-regulate activation signals mediated by non-receptor tyrosine kinase cascades through the recruitment of SHP-1. However, the exact mechanisms of LILRB4-mediated inhibition are not fully elucidated. In this study, we demonstrate high level surface expression of LILRB4 on THP-1 cells and primary peripheral blood monocytes, which profoundly inhibited production of a key pro-inflammatory cytokine (TNFalpha) induced by FcgammaRI (CD64). We also report that LILRB4 aggregated to sites of activation upon co-ligation with CD64 and that this may enhance its inhibitory effects. Cross-linking of CD64 on THP-1 cells markedly increased phosphorylation of multiple proteins including tyrosine kinases and signaling molecules (Lck, Syk, LAT, and Erk), an adaptor protein that targets protein-tyrosine kinases for degradation (c-Cbl) and a protein involved in the formation of actin cytoskeletal rearrangement (alpha-actinin-4). Co-ligation of LILRB4 considerably reduced CD64-mediated phosphorylation of Lck, Syk, LAT, Erk, and c-Cbl but not alpha-actinin-4, suggesting selective inhibition of signaling molecules. Treatment of cells with a broad-spectrum phosphatase inhibitor, sodium pervanadate (SP), significantly reversed LILRB4-mediated inhibition of TNFalpha production and protein tyrosine phosphorylation. In comparison, treatment with an SHP-1 specific inhibitor, sodium stibogluconate (SS) has no effects indicating involvement of phosphatase(s) other than SHP-1 in LILRB4 signaling. Collectively, our data show LILRB4 is a potent inhibitor of monocytes activation. This may provide a new potential therapeutic strategy for inflammatory conditions characterized by excessive TNFalpha production.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

