The localized scleroderma skin severity index and physician global assessment of disease activity: a work in progress toward development of localized scleroderma outcome measures
- PMID: 19833758
- PMCID: PMC3690503
- DOI: 10.3899/jrheum.081284
The localized scleroderma skin severity index and physician global assessment of disease activity: a work in progress toward development of localized scleroderma outcome measures
Abstract
Objective: To develop and evaluate a Localized Scleroderma (LS) Skin Severity Index (LoSSI) and global assessments' clinimetric property and effect on quality of life (QOL).
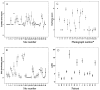
Methods: A 3-phase study was conducted. The first phase involved 15 patients with LS and 14 examiners who assessed LoSSI [surface area (SA), erythema (ER), skin thickness (ST), and new lesion/extension (N/E)] twice for inter/intrarater reliability. Patient global assessment of disease severity (PtGA-S) and Children's Dermatology Life Quality Index (CDLQI) were collected for intrarater reliability evaluation. The second phase was aimed to develop clinical determinants for physician global assessment of disease activity (PhysGA-A) and to assess its content validity. The third phase involved 2 examiners assessing LoSSI and PhysGA-A on 27 patients. Effect of training on improving reliability/validity and sensitivity to change of the LoSSI and PhysGA-A was determined.
Results: Interrater reliability was excellent for ER [intraclass correlation coefficient (ICC) 0.71], ST (ICC 0.70), LoSSI (ICC 0.80), and PhysGA-A (ICC 0.90) but poor for SA (ICC 0.35); thus, LoSSI was modified to mLoSSI. Examiners' experience did not affect the scores, but training/practice improved reliability. Intrarater reliability was excellent for ER, ST, and LoSSI (Spearman's rho = 0.71-0.89) and moderate for SA. PtGA-S and CDLQI showed good intrarater agreement (ICC 0.63 and 0.80). mLoSSI correlated moderately with PhysGA-A and PtGA-S. Both mLoSSI and PhysGA-A were sensitive to change following therapy.
Conclusion: mLoSSI and PhysGA-A are reliable and valid tools for assessing LS disease severity and show high sensitivity to detect change over time. These tools are feasible for use in routine clinical practice. They should be considered for inclusion in a core set of LS outcome measures for clinical trials.
Figures
References
-
- Torres JE, Sanchez JL. Histopathologic differentiation between localized and systemic scleroderma. Am J Dermatopathol. 1998;20:242–5. - PubMed
-
- Peterson LS, Nelson AM, Su WP, Mason T, O’Fallon WM, Gabriel SE. The epidemiology of morphea (localized scleroderma) in Olmsted County 1960–1993. J Rheumatol. 1997;24:73–80. - PubMed
-
- Peterson LS, Nelson AM, Su WP. Classification of morphea (localized scleroderma) Mayo Clin Proc. 1995;70:1068–76. - PubMed
-
- Marzano AV, Menni S, Parodi A, Borghi A, Fuligni A, Fabbri P, et al. Localized scleroderma in adults and children. Clinical and laboratory investigations on 239 cases. Eur J Dermatol. 2003;13:171–6. - PubMed
-
- Zulian F, Athreya BH, Laxer R, Nelson AM, Feitosa de Oliveira SK, Punaro MG, et al. Juvenile localized scleroderma: clinical and epidemiological features in 750 children. An international study. Rheumatology. 2006;45:614–20. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
