Oral PEG 15-20 protects the intestine against radiation: role of lipid rafts
- PMID: 19833862
- PMCID: PMC2850088
- DOI: 10.1152/ajpgi.00328.2009
Oral PEG 15-20 protects the intestine against radiation: role of lipid rafts
Abstract
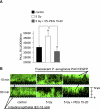
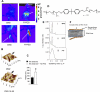
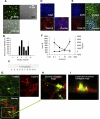
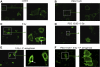
Intestinal injury following abdominal radiation therapy or accidental exposure remains a significant clinical problem that can result in varying degrees of mucosal destruction such as ulceration, vascular sclerosis, intestinal wall fibrosis, loss of barrier function, and even lethal gut-derived sepsis. We determined the ability of a high-molecular-weight polyethylene glycol-based copolymer, PEG 15-20, to protect the intestine against the early and late effects of radiation in mice and rats and to determine its mechanism of action by examining cultured rat intestinal epithelia. Rats were exposed to fractionated radiation in an established model of intestinal injury, whereby an intestinal segment is surgically placed into the scrotum and radiated daily. Radiation injury score was decreased in a dose-dependent manner in rats gavaged with 0.5 or 2.0 g/kg per day of PEG 15-20 (n = 9-13/group, P < 0.005). Complementary studies were performed in a novel mouse model of abdominal radiation followed by intestinal inoculation with Pseudomonas aeruginosa (P. aeruginosa), a common pathogen that causes lethal gut-derived sepsis following radiation. Mice mortality was decreased by 40% in mice drinking 1% PEG 15-20 (n = 10/group, P < 0.001). Parallel studies were performed in cultured rat intestinal epithelial cells treated with PEG 15-20 before radiation. Results demonstrated that PEG 15-20 prevented radiation-induced intestinal injury in rats, prevented apoptosis and lethal sepsis attributable to P. aeruginosa in mice, and protected cultured intestinal epithelial cells from apoptosis and microbial adherence and possible invasion. PEG 15-20 appeared to exert its protective effect via its binding to lipid rafts by preventing their coalescence, a hallmark feature in intestinal epithelial cells exposed to radiation.
Figures


References
-
- Baddeley AJ, Gundersen HJ, Cruz-Orive LM. Estimation of surface area from vertical sections. J Microsc 142: 259–276, 1986 - PubMed
-
- Bertz H, Auner HW, Weissinger F, Salwender HJ, Einsele H, Egerer G, Sandherr M, Schuttrumpf S, Sudhoff T, Maschmeyer G. Antimicrobial therapy of febrile complications after high-dose chemo-/radiotherapy and autologous hematopoietic stem cell transplantation—guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann Hematol 82, Suppl 2: S167–S174, 2003 - PubMed
-
- Bionda C, Hadchity E, Alphonse G, Chapet O, Rousson R, Rodriguez-Lafrasse C, Ardail D. Radioresistance of human carcinoma cells is correlated to a defect in raft membrane clustering. Free Radic Biol Med 43: 681–694, 2007 - PubMed
-
- Brook I, Elliott TB, Ledney GD, Knudson GB. Management of postirradiation sepsis. Mil Med 167: 105–106, 2002 - PubMed
-
- Brook I, Elliott TB, Ledney GD, Shoemaker MO, Knudson GB. Management of postirradiation infection: lessons learned from animal models. Mil Med 169: 194–197, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

