Computational design of ligand binding is not a solved problem
- PMID: 19833875
- PMCID: PMC2773959
- DOI: 10.1073/pnas.0907950106
Computational design of ligand binding is not a solved problem
Abstract
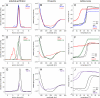
Computational design has been very successful in recent years: multiple novel ligand binding proteins as well as enzymes have been reported. We wanted to know in molecular detail how precise the predictions of the interactions of protein and ligands are. Therefore, we performed a structural analysis of a number of published receptors designed onto the periplasmic binding protein scaffold that were reported to bind to the new ligands with nano- to micromolar affinities. It turned out that most of these designed proteins are not suitable for structural studies due to instability and aggregation. However, we were able to solve the crystal structure of an arabinose binding protein designed to bind serotonin to 2.2 A resolution. While crystallized in the presence of an excess of serotonin, the protein is in an open conformation with no serotonin bound, although the side-chain conformations in the empty binding pocket are very similar to the conformations predicted. During subsequent characterization using isothermal titration calorimetry, CD, and NMR spectroscopy, no indication of binding could be detected for any of the tested designed receptors, whereas wild-type proteins bound their ligands as expected. We conclude that although the computational prediction of side-chain conformations appears to be working, it does not necessarily confer binding as expected. Hence, the computational design of ligand binding is not a solved problem and needs to be revisited.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

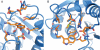


References
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

