Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue
- PMID: 19833890
- PMCID: PMC2797918
- DOI: 10.2337/db09-0929
Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue
Abstract
Objective: White adipose tissue (WAT) and brown adipose tissue (BAT) play distinct roles in adaptation to changes in nutrient availability, with WAT serving as an energy store and BAT regulating thermogenesis. We previously showed that mice maintained on a leucine-deficient diet unexpectedly experienced a dramatic reduction in abdominal fat mass. The cellular mechanisms responsible for this loss, however, are unclear. The goal of current study is to investigate possible mechanisms.
Research design and methods: Male C57BL/6J mice were fed either control, leucine-deficient, or pair-fed diets for 7 days. Changes in metabolic parameters and expression of genes and proteins related to lipid metabolism were analyzed in WAT and BAT.
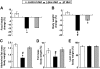
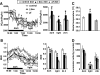
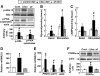
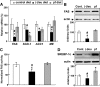
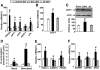
Results: We found that leucine deprivation for 7 days increases oxygen consumption, suggesting increased energy expenditure. We also observed increases in lipolysis and expression of beta-oxidation genes and decreases in expression of lipogenic genes and activity of fatty acid synthase in WAT, consistent with increased use and decreased synthesis of fatty acids, respectively. Furthermore, we observed that leucine deprivation increases expression of uncoupling protein (UCP)-1 in BAT, suggesting increased thermogenesis.
Conclusions: We show for the first time that elimination of dietary leucine produces significant metabolic changes in WAT and BAT. The effect of leucine deprivation on UCP1 expression is a novel and unexpected observation and suggests that the observed increase in energy expenditure may reflect an increase in thermogenesis in BAT. Further investigation will be required to determine the relative contribution of UCP1 upregulation and thermogenesis in BAT to leucine deprivation-stimulated fat loss.
Figures

References
-
- Hofbauer KG: Molecular pathways to obesity. Int J Obes Relat Metab Disord 2002;26(Suppl. 2):S18–S27 - PubMed
-
- Kershaw EE, Flier JS: Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004;89:2548–2556 - PubMed
-
- Fernández-López JA, Remesar X, Foz M, Alemany M: Pharmacological approaches for the treatment of obesity. Drugs 2002;62:915–944 - PubMed
-
- Layman DK, Walker DA: Potential importance of leucine in treatment of obesity and the metabolic syndrome. J Nutr 2006;136:319S–323S - PubMed
-
- van Dam RM, Seidell JC: Carbohydrate intake and obesity. Eur J Clin Nutr 2007;61(Suppl. 1):S75–S99 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

