Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands
- PMID: 19833897
- PMCID: PMC2797929
- DOI: 10.2337/db09-0801
Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands
Abstract
Objective: RAGE interacts with the endogenous ligands S100 calgranulins and high mobility group box 1 (HMGB1) to induce inflammation. Since hyperglycemia-induced reactive oxygen species (ROS) activate many pathways of diabetic tissue damage, the effect of these ROS on RAGE and RAGE ligand expression was evaluated.
Research design and methods: Expression of RAGE, S100A8, S100A12, and HMGB1 was evaluated in human aortic endothelial cells (HAECs) incubated in normal glucose, high glucose, and high glucose after overexpression of either uncoupling protein 1 (UCP1), superoxide dismutase 2 (SOD2), or glyoxalase 1 (GLO1). Expression was also evaluated in normal glucose after knockdown of GLO1. Expression was next evaluated in high glucose after knockdown of nuclear factor (NF)-kappaB p65 (RAGE) and after knockdown of activated protein-1 (AP-1) (S100A8, S100A12, and HMGB1), and chromatin immunoprecipitation (ChIP) was performed +/- GLO1 overexpression for NFkappaB p65 (RAGE promoter) and AP-1 (S100A8, S100A12, and HMGB1 promoters). Finally, endothelial cells from nondiabetic mice, STZ diabetic mice, and STZ diabetic mice treated with the superoxide dismutase mimetic Mn(III)tetrakis(4-benzoic acid)porphyrin chloride (MnTBAP) were evaluated.
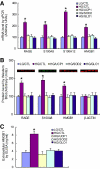
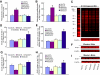
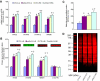
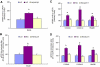
Results: High glucose increased RAGE, S100A8, S100A12, and HMGB1 expression, which was normalized by overexpression of UCP1, SOD2, or GLO1. GLO1 knockdown mimicked the effect of high glucose, and in high glucose, overexpression of GLO1 normalized increased binding of NFkappaB p65 and AP-1. Diabetes increased RAGE, S100A8, and HMGB1 expression, and MnTBAP treatment normalized this.
Conclusions: These results show that hyperglycemia-induced ROS production increases expression of RAGE and RAGE ligands. This effect is mediated by ROS-induced methylglyoxal, the major substrate of glyoxalase 1.
Figures
Similar articles
-
Gene doubling increases glyoxalase 1 expression in RAGE knockout mice.Biochim Biophys Acta Gen Subj. 2020 Jan;1864(1):129438. doi: 10.1016/j.bbagen.2019.129438. Epub 2019 Sep 14. Biochim Biophys Acta Gen Subj. 2020. PMID: 31526867
-
Glycine Suppresses AGE/RAGE Signaling Pathway and Subsequent Oxidative Stress by Restoring Glo1 Function in the Aorta of Diabetic Rats and in HUVECs.Oxid Med Cell Longev. 2019 Mar 3;2019:4628962. doi: 10.1155/2019/4628962. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 30944692 Free PMC article.
-
Acute glucose fluctuation promotes RAGE expression via reactive oxygen species‑mediated NF‑κB activation in rat podocytes.Mol Med Rep. 2021 May;23(5):330. doi: 10.3892/mmr.2021.11969. Epub 2021 Mar 24. Mol Med Rep. 2021. PMID: 33760170 Free PMC article.
-
Perspectives on RAGE signaling and its role in cardiovascular disease.Am J Med Genet A. 2013 Nov;161A(11):2750-5. doi: 10.1002/ajmg.a.36181. Epub 2013 Oct 3. Am J Med Genet A. 2013. PMID: 24123885 Review.
-
Expatiating the molecular approaches of HMGB1 in diabetes mellitus: Highlighting signalling pathways via RAGE and TLRs.Mol Biol Rep. 2021 Feb;48(2):1869-1881. doi: 10.1007/s11033-020-06130-x. Epub 2021 Jan 21. Mol Biol Rep. 2021. PMID: 33479829 Review.
Cited by
-
Beta-adrenergic agonism protects mitochondrial metabolism in the pancreatectomised rat heart.Sci Rep. 2024 Aug 21;14(1):19383. doi: 10.1038/s41598-024-70335-4. Sci Rep. 2024. PMID: 39169098 Free PMC article.
-
S100A8/A9-activated IFNγ+ NK cells trigger β-cell necroptosis in hepatitis B virus-associated liver cirrhosis.Cell Mol Life Sci. 2024 Aug 12;81(1):345. doi: 10.1007/s00018-024-05365-2. Cell Mol Life Sci. 2024. PMID: 39133305 Free PMC article.
-
The link between metabolic abnormalities and endothelial dysfunction in type 2 diabetes: an update.Basic Res Cardiol. 2012 Jan;107(1):237. doi: 10.1007/s00395-011-0237-1. Epub 2011 Dec 22. Basic Res Cardiol. 2012. PMID: 22189563 Free PMC article. Review.
-
Systemic RAGE ligands are upregulated in tuberculosis individuals with diabetes co-morbidity and modulated by anti-tuberculosis treatment and metformin therapy.BMC Infect Dis. 2019 Dec 9;19(1):1039. doi: 10.1186/s12879-019-4648-1. BMC Infect Dis. 2019. PMID: 31818258 Free PMC article.
-
2013 Russell Ross memorial lecture in vascular biology: cellular and molecular mechanisms of diabetes mellitus-accelerated atherosclerosis.Arterioscler Thromb Vasc Biol. 2014 Apr;34(4):705-14. doi: 10.1161/ATVBAHA.113.301928. Arterioscler Thromb Vasc Biol. 2014. PMID: 24665124 Free PMC article.
References
-
- Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM: RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell 1999;97:889–901 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

