Public perspectives on informed consent for biobanking
- PMID: 19833988
- PMCID: PMC2775766
- DOI: 10.2105/AJPH.2008.157099
Public perspectives on informed consent for biobanking
Abstract
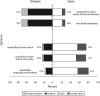
The National Institutes of Health and other federal health agencies are considering establishing a national biobank to study the roles of genes and environment in health. We assessed the public's attitudes toward the proposed biobank, including preferences for providing informed consent. Sixteen focus groups were conducted, and themes arising from the focus groups were tested in a large, representative survey (n=4659) of the general population. Our research demonstrates that when considering participating in a genomic biobank, individuals want ongoing choices and control over access to their samples and information.
Figures
References
-
- National Bioethics Advisory Commission Research involving human biological materials: Ethical issues and policy guidance. Available at: http://www.bioethics.gov/reports/past_commissions/nbac_human_part.pdf. Accessed June 29, 2009
-
- Berg JW, Appelbaum PS, Lidz CW, Parker LS. Informed Consent: Legal Theory and Clinical Practice 2nd ed New York, NY: Oxford University Press; 2001
-
- National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research Belmont report: ethical principles and guidelines for the protection of human subjects of research. Available at: http://ohsr.od.nih.gov/guidelines/belmont.html. Accessed June 29, 2009 - PubMed
-
- World Health Organization Proposed international guidelines on ethical issues in medical genetics and genetic services. Available at: http://www.who.int/genomics/publications/en/index1.html. Accessed June 29, 2009 - PubMed
-
- 45 CFR §46.116(a) (1991)
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

