Glycosaminoglycan-mediated loss of cathepsin K collagenolytic activity in MPS I contributes to osteoclast and growth plate abnormalities
- PMID: 19834056
- PMCID: PMC2774069
- DOI: 10.2353/ajpath.2009.090211
Glycosaminoglycan-mediated loss of cathepsin K collagenolytic activity in MPS I contributes to osteoclast and growth plate abnormalities
Abstract
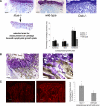
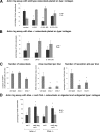
Mucopolysaccharidoses are a group of lysosomal storage diseases characterized by the build-up of glycosaminoglycans (GAGs) and severe skeletal abnormalities. As GAGs can regulate the collagenolytic activity of the major osteoclastic protease cathepsin K, we investigated the presence and activity of cathepsin K and its co-localization with GAGs in mucopolysaccharidosis (MPS) type I bone. The most dramatic difference between MPS I and wild-type mice was an increase in the amount of cartilage in the growth plates in MPS I bones. Though the number of cathepsin K-expressing osteoclasts was increased in MPS I mice, these mice revealed a significant reduction in cathepsin K-mediated cartilage degradation. As excess heparan and dermatan sulfates inhibit type II collagen degradation by cathepsin K and the spatial overlap between cathepsin K and heparan sulfate strongly increased in MPS I mice, the build up of subepiphyseal cartilage is speculated to be a direct consequence of cathepsin K inhibition by MPS I-associated GAGs. Moreover, isolated MPS I and Ctsk(-/-) osteoclasts displayed fewer actin rings and formed fewer resorption pits on dentine disks, as compared with wild-type cells. These results suggest that the accumulation of GAGs in murine MPS I bone has an inhibitory effect on cathepsin K activity, resulting in impaired osteoclast activity and decreased cartilage resorption, which may contribute to the bone pathology seen in MPS diseases.
Figures


References
-
- Neufeld EF, Muenzer J. The Mucopolysaccharidoses. New York: McGraw-Hill,; 2001:pp 3419–3428.
-
- Burrow TA, Hopkin RJ, Leslie ND, Tinkle BT, Grabowski GA. Enzyme reconstitution/replacement therapy for lysosomal storage diseases. Curr Opin Pediatr. 2007;19:628–635. - PubMed
-
- Yasuda Y, Kaleta J, Bromme D. The role of cathepsins in osteoporosis and arthritis: rationale for the design of new therapeutics. Adv Drug Deliv Rev. 2005;57:973–993. - PubMed
-
- Gelb BD, Shi GP, Chapman HA, Desnick RJ. Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science. 1996;273:1236–1238. - PubMed
-
- Everts V, Hou WS, Rialland X, Tigchelaar W, Saftig P, Bromme D, Gelb BD, Beertsen W. Cathepsin K deficiency in pycnodysostosis results in accumulation of non-digested phagocytosed collagen in fibroblasts. Calcif Tissue Int. 2003;73:380–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

