Cripto-1 is required for hypoxia to induce cardiac differentiation of mouse embryonic stem cells
- PMID: 19834060
- PMCID: PMC2774077
- DOI: 10.2353/ajpath.2009.090218
Cripto-1 is required for hypoxia to induce cardiac differentiation of mouse embryonic stem cells
Abstract
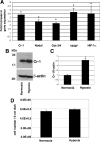
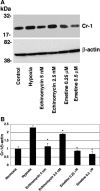
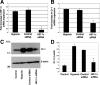
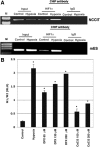
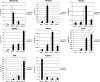
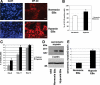
Cripto-1 is a membrane-bound protein that is highly expressed in embryonic stem cells and in human tumors. In the present study, we investigated the effect of low levels of oxygen, which occurs naturally in rapidly growing tissues, on Cripto-1 expression in mouse embryonic stem (mES) cells and in human embryonal carcinoma cells. During hypoxia, Cripto-1 expression levels were significantly elevated in mES cells and in Ntera-2 or NCCIT human embryonal carcinoma cells, as compared with cells growing with normal oxygen levels. The transcription factor hypoxia-inducible factor-1alpha directly regulated Cripto-1 expression by binding to hypoxia-responsive elements within the promoter of mouse and human Cripto-1 genes in mES and NCCIT cells, respectively. Furthermore, hypoxia modulated differentiation of mES cells by enhancing formation of beating cardiomyocytes as compared with mES cells that were differentiated under normoxia. However, hypoxia failed to induce differentiation of mES cells into cardiomyocytes in the absence of Cripto-1 expression, demonstrating that Cripto-1 is required for hypoxia to fully differentiate mES cells into cardiomyocytes. Finally, cardiac tissue samples derived from patients who had suffered ischemic heart disease showed a dramatic increase in Cripto-1 expression as compared with nonischemic heart tissue samples, suggesting that hypoxia may also regulate Cripto-1 in vivo.
Figures
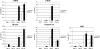

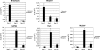



References
-
- Bianco C, Strizzi L, Normanno N, Khan N, Salomon DS. Cripto-1: an oncofetal gene with many faces. Curr Top Dev Biol. 2005;67:85–133. - PubMed
-
- Schier AF. Nodal signaling in vertebrate development. Annu Rev Cell Dev Biol. 2003;19:589–621. - PubMed
-
- Dono R, Scalera L, Pacifico F, Acampora D, Persico MG, Simeone A. The murine cripto gene: expression during mesoderm induction and early heart morphogenesis. Development. 1993;118:1157–1168. - PubMed
-
- Xu C, Liguori G, Persico MG, Adamson ED. Abrogation of the Cripto gene in mouse leads to failure of postgastrulation morphogenesis and lack of differentiation of cardiomyocytes. Development. 1999;126:483–494. - PubMed
-
- Xu C, Liguori G, Adamson ED, Persico MG. Specific arrest of cardiogenesis in cultured embryonic stem cells lacking Cripto-1. Dev Biol. 1998;196:237–247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

