Disruption of PPARgamma signaling results in mouse prostatic intraepithelial neoplasia involving active autophagy
- PMID: 19834493
- PMCID: PMC2821953
- DOI: 10.1038/cdd.2009.148
Disruption of PPARgamma signaling results in mouse prostatic intraepithelial neoplasia involving active autophagy
Abstract
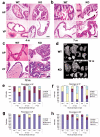
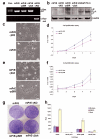
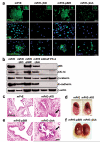
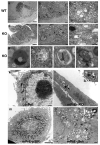
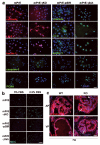
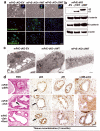
Peroxisome proliferator-activated receptor-gamma (PPARgamma) regulates the interface between cellular lipid metabolism, redox status and organelle differentiation. Conditional prostatic epithelial knockout of PPARgamma in mice resulted in focal hyperplasia which developed into mouse prostatic intraepithelial neoplasia (mPIN). The grade of PIN became more severe with time. Electron microscopy (EM) showed accumulated secondary lysosomes containing cellular organelles and debris suggestive of autophagy. Consistent with this analysis the autophagy marker LC-3 was found to be upregulated in areas of PIN in PPARgamma KO tissues. We selectively knocked down PPARgamma2 isoform in wild-type mouse prostatic epithelial cells and examined the consequences of this in a tissue recombination model. Histopathologically grafted tissues resembled the conditional PPARgamma KO mouse prostates. EM studies of PPARgamma- and PPARgamma2-deficient epithelial cells in vitro were suggestive of autophagy, consistent with the prostatic tissue analysis. This was confirmed by examining expression of beclin-1 and LC-3. Gene expression profiling in PPARgamma-/gamma2-deficient cells indicated a major dysregulation of cell cycle control and metabolic signaling networks related to peroxisomal and lysosomal maturation, lipid oxidation and degradation. The putative autophagic phenotypes of PPARgamma-deficient cells could be rescued by re-expression of either gamma1 or gamma2 isoform. We conclude that disruption of PPARgamma signaling results in autophagy and oxidative stress during mPIN pathogenesis.
Figures

Similar articles
-
Autophagy in nuclear receptor PPARgamma-deficient mouse prostatic carcinogenesis.Autophagy. 2010 Jan;6(1):175-6. doi: 10.4161/auto.6.1.10700. Epub 2010 Jan 18. Autophagy. 2010. PMID: 20009560 Review.
-
PPARγ isoforms differentially regulate metabolic networks to mediate mouse prostatic epithelial differentiation.Cell Death Dis. 2012 Aug 9;3(8):e361. doi: 10.1038/cddis.2012.99. Cell Death Dis. 2012. PMID: 22874998 Free PMC article.
-
Stromal activation associated with development of prostate cancer in prostate-targeted fibroblast growth factor 8b transgenic mice.Neoplasia. 2010 Nov;12(11):915-27. doi: 10.1593/neo.10776. Neoplasia. 2010. PMID: 21076617 Free PMC article.
-
MYC overexpression induces prostatic intraepithelial neoplasia and loss of Nkx3.1 in mouse luminal epithelial cells.PLoS One. 2010 Feb 25;5(2):e9427. doi: 10.1371/journal.pone.0009427. PLoS One. 2010. PMID: 20195545 Free PMC article.
-
Neuroendocrine differentiation in the precursors of prostate cancer.Eur Urol. 1996;30(2):185-90. doi: 10.1159/000474168. Eur Urol. 1996. PMID: 8875199 Review.
Cited by
-
Rosiglitazone protects against palmitate-induced pancreatic beta-cell death by activation of autophagy via 5'-AMP-activated protein kinase modulation.Endocrine. 2013 Aug;44(1):87-98. doi: 10.1007/s12020-012-9826-5. Epub 2012 Oct 31. Endocrine. 2013. PMID: 23109223
-
Review of Prostate Anatomy and Embryology and the Etiology of Benign Prostatic Hyperplasia.Urol Clin North Am. 2016 Aug;43(3):279-88. doi: 10.1016/j.ucl.2016.04.012. Urol Clin North Am. 2016. PMID: 27476121 Free PMC article. Review.
-
Androgens promote prostate cancer cell growth through induction of autophagy.Mol Endocrinol. 2013 Feb;27(2):280-95. doi: 10.1210/me.2012-1260. Epub 2012 Dec 18. Mol Endocrinol. 2013. PMID: 23250485 Free PMC article.
-
Asiatic acid mitigates testosterone-induced benign prostatic hyperplasia in rats via activation of PPAR-γ.Naunyn Schmiedebergs Arch Pharmacol. 2025 Feb;398(2):1991-2001. doi: 10.1007/s00210-024-03405-z. Epub 2024 Sep 2. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39222244
-
The Androgen Receptor Regulates PPARγ Expression and Activity in Human Prostate Cancer Cells.J Cell Physiol. 2016 Dec;231(12):2664-72. doi: 10.1002/jcp.25368. Epub 2016 Mar 14. J Cell Physiol. 2016. PMID: 26945682 Free PMC article.
References
-
- Yu K, Bayona W, Kallen CB, Harding HP, Ravera CP, McMahon G, et al. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J Biol Chem. 1995 Oct 13;270(41):23975–23983. - PubMed
-
- Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes & development. 1994 May 15;8(10):1224–1234. - PubMed
-
- Michalik L, Desvergne B, Dreyer C, Gavillet M, Laurini RN, Wahli W. PPAR expression and function during vertebrate development. The International journal of developmental biology. 2002 Jan;46(1):105–114. - PubMed
-
- Mao-Qiang M, Fowler AJ, Schmuth M, Lau P, Chang S, Brown BE, et al. Peroxisome-proliferator-activated receptor (PPAR)-gamma activation stimulates keratinocyte differentiation. J Invest Dermatol. 2004 Aug;123(2):305–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK58404/DK/NIDDK NIH HHS/United States
- P30 CA68485/CA/NCI NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- R01 CA059705/CA/NCI NIH HHS/United States
- R01 DK67049/DK/NIDDK NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- DK20539/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- R01 CA59705/CA/NCI NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
- R01 DK055748/DK/NIDDK NIH HHS/United States
- R56 DK067049/DK/NIDDK NIH HHS/United States
- P30 DK58404/DK/NIDDK NIH HHS/United States
- R01 CA113392/CA/NCI NIH HHS/United States
- R01 DK067049/DK/NIDDK NIH HHS/United States
- P30 EY08126/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

