Disruption of PPARgamma signaling results in mouse prostatic intraepithelial neoplasia involving active autophagy
- PMID: 19834493
- PMCID: PMC2821953
- DOI: 10.1038/cdd.2009.148
Disruption of PPARgamma signaling results in mouse prostatic intraepithelial neoplasia involving active autophagy
Abstract
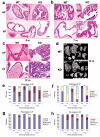
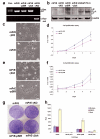
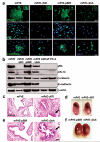
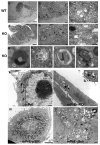
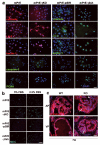
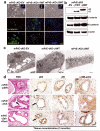
Peroxisome proliferator-activated receptor-gamma (PPARgamma) regulates the interface between cellular lipid metabolism, redox status and organelle differentiation. Conditional prostatic epithelial knockout of PPARgamma in mice resulted in focal hyperplasia which developed into mouse prostatic intraepithelial neoplasia (mPIN). The grade of PIN became more severe with time. Electron microscopy (EM) showed accumulated secondary lysosomes containing cellular organelles and debris suggestive of autophagy. Consistent with this analysis the autophagy marker LC-3 was found to be upregulated in areas of PIN in PPARgamma KO tissues. We selectively knocked down PPARgamma2 isoform in wild-type mouse prostatic epithelial cells and examined the consequences of this in a tissue recombination model. Histopathologically grafted tissues resembled the conditional PPARgamma KO mouse prostates. EM studies of PPARgamma- and PPARgamma2-deficient epithelial cells in vitro were suggestive of autophagy, consistent with the prostatic tissue analysis. This was confirmed by examining expression of beclin-1 and LC-3. Gene expression profiling in PPARgamma-/gamma2-deficient cells indicated a major dysregulation of cell cycle control and metabolic signaling networks related to peroxisomal and lysosomal maturation, lipid oxidation and degradation. The putative autophagic phenotypes of PPARgamma-deficient cells could be rescued by re-expression of either gamma1 or gamma2 isoform. We conclude that disruption of PPARgamma signaling results in autophagy and oxidative stress during mPIN pathogenesis.
Figures

References
-
- Yu K, Bayona W, Kallen CB, Harding HP, Ravera CP, McMahon G, et al. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J Biol Chem. 1995 Oct 13;270(41):23975–23983. - PubMed
-
- Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes & development. 1994 May 15;8(10):1224–1234. - PubMed
-
- Michalik L, Desvergne B, Dreyer C, Gavillet M, Laurini RN, Wahli W. PPAR expression and function during vertebrate development. The International journal of developmental biology. 2002 Jan;46(1):105–114. - PubMed
-
- Mao-Qiang M, Fowler AJ, Schmuth M, Lau P, Chang S, Brown BE, et al. Peroxisome-proliferator-activated receptor (PPAR)-gamma activation stimulates keratinocyte differentiation. J Invest Dermatol. 2004 Aug;123(2):305–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK58404/DK/NIDDK NIH HHS/United States
- P30 CA68485/CA/NCI NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- R01 CA059705/CA/NCI NIH HHS/United States
- R01 DK67049/DK/NIDDK NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- DK20539/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- R01 CA59705/CA/NCI NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
- R01 DK055748/DK/NIDDK NIH HHS/United States
- R56 DK067049/DK/NIDDK NIH HHS/United States
- P30 DK58404/DK/NIDDK NIH HHS/United States
- R01 CA113392/CA/NCI NIH HHS/United States
- R01 DK067049/DK/NIDDK NIH HHS/United States
- P30 EY08126/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

