Targeted induction of endoplasmic reticulum stress induces cartilage pathology
- PMID: 19834559
- PMCID: PMC2757901
- DOI: 10.1371/journal.pgen.1000691
Targeted induction of endoplasmic reticulum stress induces cartilage pathology
Abstract
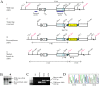
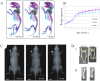
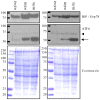
Pathologies caused by mutations in extracellular matrix proteins are generally considered to result from the synthesis of extracellular matrices that are defective. Mutations in type X collagen cause metaphyseal chondrodysplasia type Schmid (MCDS), a disorder characterised by dwarfism and an expanded growth plate hypertrophic zone. We generated a knock-in mouse model of an MCDS-causing mutation (COL10A1 p.Asn617Lys) to investigate pathogenic mechanisms linking genotype and phenotype. Mice expressing the collagen X mutation had shortened limbs and an expanded hypertrophic zone. Chondrocytes in the hypertrophic zone exhibited endoplasmic reticulum (ER) stress and a robust unfolded protein response (UPR) due to intracellular retention of mutant protein. Hypertrophic chondrocyte differentiation and osteoclast recruitment were significantly reduced indicating that the hypertrophic zone was expanded due to a decreased rate of VEGF-mediated vascular invasion of the growth plate. To test directly the role of ER stress and UPR in generating the MCDS phenotype, we produced transgenic mouse lines that used the collagen X promoter to drive expression of an ER stress-inducing protein (the cog mutant of thyroglobulin) in hypertrophic chondrocytes. The hypertrophic chondrocytes in this mouse exhibited ER stress with a characteristic UPR response. In addition, the hypertrophic zone was expanded, gene expression patterns were disrupted, osteoclast recruitment to the vascular invasion front was reduced, and long bone growth decreased. Our data demonstrate that triggering ER stress per se in hypertrophic chondrocytes is sufficient to induce the essential features of the cartilage pathology associated with MCDS and confirm that ER stress is a central pathogenic factor in the disease mechanism. These findings support the contention that ER stress may play a direct role in the pathogenesis of many connective tissue disorders associated with the expression of mutant extracellular matrix proteins.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
-
- Aszódi A, Bateman JF, Gustafsson E, Boot-Handford R, Fässler R. Mammalian skeletogenesis and extracellular matrix: What can we learn from knockout mice? Cell Structure and Function. 2000;25:71–82. - PubMed
-
- Chan D, Jacenko O. Phenotypic and biochemical consequences of collagen X mutations in mice and humans. Matrix Biol. 1998;17:169–184. - PubMed
-
- Bateman JF, Wilson R, Freddi S, Lamande S, Savarirayan R. Mutations of COL10A1 in Schmid Metaphyseal Chondrodysplasia. Hum Mutation. 2005;25:525–534. - PubMed
-
- Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

