Enteric neural crest differentiation in ganglioneuromas implicates Hedgehog signaling in peripheral neuroblastic tumor pathogenesis
- PMID: 19834598
- PMCID: PMC2759000
- DOI: 10.1371/journal.pone.0007491
Enteric neural crest differentiation in ganglioneuromas implicates Hedgehog signaling in peripheral neuroblastic tumor pathogenesis
Erratum in
- PLoS One. 2014 Jul 25;9(7):e103881. Shirazi, Arash [corrected to Shiraz, Ashton]
Abstract
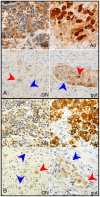
Peripheral neuroblastic tumors (PNTs) share a common origin in the sympathetic nervous system, but manifest variable differentiation and growth potential. Malignant neuroblastoma (NB) and benign ganglioneuroma (GN) stand at opposite ends of the clinical spectrum. We hypothesize that a common PNT progenitor is driven to variable differentiation by specific developmental signaling pathways. To elucidate developmental pathways that direct PNTs along the differentiation spectrum, we compared the expression of genes related to neural crest development in GN and NB. In GNs, we found relatively low expression of sympathetic markers including adrenergic biosynthesis enzymes, indicating divergence from sympathetic fate. In contrast, GNs expressed relatively high levels of enteric neuropeptides and key constituents of the Hedgehog (HH) signaling pathway, including Dhh, Gli1 and Gli3. Predicted HH targets were also differentially expressed in GN, consistent with transcriptional response to HH signaling. These findings indicate that HH signaling is specifically active in GN. Together with the known role of HH activity in enteric neural development, these findings further suggested a role for HH activity in directing PNTs away from the sympathetic lineage toward a benign GN phenotype resembling enteric ganglia. We tested the potential for HH signaling to advance differentiation in PNTs by transducing NB cell lines with Gli1 and determining phenotypic and transcriptional response. Gli1 inhibited proliferation of NB cells, and induced a pattern of gene expression that resembled the differential pattern of gene expression of GN, compared to NB (p<0.00001). Moreover, the transcriptional response of SY5Y cells to Gli1 transduction closely resembled the transcriptional response to the differentiation agent retinoic acid (p<0.00001). Notably, Gli1 did not induce N-MYC expression in neuroblastoma cells, but strongly induced RET, a known mediator of RA effect. The decrease in NB cell proliferation induced by Gli1, and the similarity in the patterns of gene expression induced by Gli1 and by RA, corroborated by closely matched gene sets in GN tumors, all support a model in which HH signaling suppresses PNT growth by promoting differentiation along alternative neural crest pathways.
Conflict of interest statement
Figures


References
-
- Park JR, Eggert A, Caron H. Neuroblastoma: biology, prognosis, and treatment. Pediatr Clin North Am. 2008;55:97–120, x. - PubMed
-
- Griffin ME, Bolande RP. Familial neuroblastoma with regression and maturation to ganglioneurofibroma. Pediatrics. 1969;43:377–382. - PubMed
-
- Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med. 1999;341:1165–1173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

