Sequence and structure signatures of cancer mutation hotspots in protein kinases
- PMID: 19834613
- PMCID: PMC2759519
- DOI: 10.1371/journal.pone.0007485
Sequence and structure signatures of cancer mutation hotspots in protein kinases
Abstract
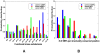
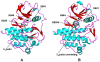
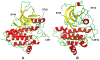
Protein kinases are the most common protein domains implicated in cancer, where somatically acquired mutations are known to be functionally linked to a variety of cancers. Resequencing studies of protein kinase coding regions have emphasized the importance of sequence and structure determinants of cancer-causing kinase mutations in understanding of the mutation-dependent activation process. We have developed an integrated bioinformatics resource, which consolidated and mapped all currently available information on genetic modifications in protein kinase genes with sequence, structure and functional data. The integration of diverse data types provided a convenient framework for kinome-wide study of sequence-based and structure-based signatures of cancer mutations. The database-driven analysis has revealed a differential enrichment of SNPs categories in functional regions of the kinase domain, demonstrating that a significant number of cancer mutations could fall at structurally equivalent positions (mutational hotspots) within the catalytic core. We have also found that structurally conserved mutational hotspots can be shared by multiple kinase genes and are often enriched by cancer driver mutations with high oncogenic activity. Structural modeling and energetic analysis of the mutational hotspots have suggested a common molecular mechanism of kinase activation by cancer mutations, and have allowed to reconcile the experimental data. According to a proposed mechanism, structural effect of kinase mutations with a high oncogenic potential may manifest in a significant destabilization of the autoinhibited kinase form, which is likely to drive tumorigenesis at some level. Structure-based functional annotation and prediction of cancer mutation effects in protein kinases can facilitate an understanding of the mutation-dependent activation process and inform experimental studies exploring molecular pathology of tumorigenesis.
Conflict of interest statement
Figures

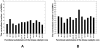






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

