Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts
- PMID: 19834619
- PMCID: PMC2759556
- DOI: 10.1371/journal.pone.0007475
Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts
Abstract
Background: The processes that drive fibrotic diseases are complex and include an influx of peripheral blood monocytes that can differentiate into fibroblast-like cells called fibrocytes. Monocytes can also differentiate into other cell types, such as tissue macrophages. The ability to discriminate between monocytes, macrophages, fibrocytes, and fibroblasts in fibrotic lesions could be beneficial in identifying therapies that target either stromal fibroblasts or fibrocytes.
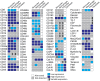
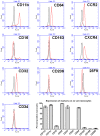
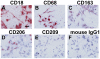
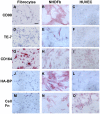
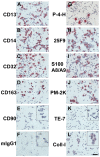
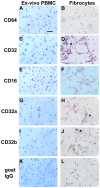
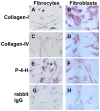
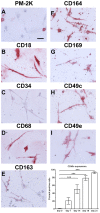
Methodology/principal findings: We have identified markers that discriminate between human peripheral blood monocytes, tissue macrophages, fibrocytes, and fibroblasts. Amongst these four cell types, only peripheral blood monocytes express the combination of CD45RO, CD93, and S100A8/A9; only macrophages express the combination of CD45RO, 25F9, S100A8/A9, and PM-2K; only fibrocytes express the combination of CD45RO, 25F9, and S100A8/A9, but not PM-2K; and only fibroblasts express the combination of CD90, cellular fibronectin, hyaluronan, and TE-7. These markers are effective both in vitro and in sections from human lung. We found that markers such as CD34, CD68, and collagen do not effectively discriminate between the four cell types. In addition, IL-4, IL-12, IL-13, IFN-gamma, and SAP differentially regulate the expression of CD32, CD163, CD172a, and CD206 on both macrophages and fibrocytes. Finally, CD49c (alpha3 integrin) expression identifies a subset of fibrocytes, and this subset increases with time in culture.
Conclusions/significance: These results suggest that discrimination of monocytes, macrophages, fibrocytes, and fibroblasts in fibrotic lesions is possible, and this may allow for an assessment of fibrocytes in fibrotic diseases.
Conflict of interest statement
Figures







References
-
- Martin P. Wound Healing—Aiming for Perfect Skin Regeneration. Science. 1997;276:75–81. - PubMed
-
- Singer AJ, Clark RA. Cutaneous wound healing. New England Journal Of Medicine. 1999;341:738–746. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
-
- Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells. 2007;25:2896–2902. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

