Regulation of phenylacetic acid degradation genes of Burkholderia cenocepacia K56-2
- PMID: 19835630
- PMCID: PMC2770484
- DOI: 10.1186/1471-2180-9-222
Regulation of phenylacetic acid degradation genes of Burkholderia cenocepacia K56-2
Abstract
Background: Metabolically versatile soil bacteria Burkholderia cepacia complex (Bcc) have emerged as opportunistic pathogens, especially of cystic fibrosis (CF). Previously, we initiated the characterization of the phenylacetic acid (PA) degradation pathway in B. cenocepacia, a member of the Bcc, and demonstrated the necessity of a functional PA catabolic pathway for full virulence in Caenorhabditis elegans. In this study, we aimed to characterize regulatory elements and nutritional requirements that control the PA catabolic genes in B. cenocepacia K56-2.
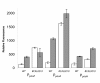
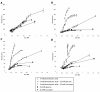
Results: Translational fusions of the PA degradation gene promoters with eGFP were constructed and introduced in B. cenocepacia K56-2. eGFP expression was observed when the reporter strains were grown in minimal media containing glycerol and PA or other compounds expected to proceed through the PA pathway, and in synthetic CF medium (SCFM). Addition of succinate or glucose to the PA containing medium repressed eGFP expression. To show that BCAL0210, a putative TetR-type regulator gene encodes a regulator for the PA genes in B. cenocepacia, we developed a BCAL0210 insertional mutant reporter strain. Results show that these strains exhibit fluorescence regardless of the presence of PA in the culture.
Conclusion: The PA catabolic genes of B. cenocepacia K56-2 are induced by PA and other related compounds, are negatively regulated by PaaR (named herein), a TetR-type regulator, and are subjected to catabolic repression by glucose and succinate. As the PA catabolic pathway of B. cenocepacia appears to be induced during growth in synthetic cystic fibrosis medium (SCFM), further research is necessary to determine the relevance of this pathway in CF-like conditions and in other host-pathogen interactions.
Figures
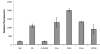


References
-
- Vanlaere E, Lipuma JJ, Baldwin A, Henry D, De Brandt E, Mahenthiralingam E, Speert D, Dowson C, Vandamme P. Burkholderia latens sp. nov., Burkholderia diffusa sp. nov., Burkholderia arboris sp. nov., Burkholderia seminalis sp. nov. and Burkholderia metallica sp. nov., novel species within the Burkholderia cepacia complex. Int J Syst Evol Microbiol. 2008;58(Pt 7):1580–1590. doi: 10.1099/ijs.0.65634-0. - DOI - PubMed
-
- Holden MT, Seth-Smith HM, Crossman LC, Sebaihia M, Bentley SD, Cerdeno-Tarraga AM, Thomson NR, Bason N, Quail MA, Sharp S, Cherevach I, Churcher C, Goodhead I, Hauser H, Holroyd N, Mungall K, Scott P, Walker D, White B, Rose H, Iversen P, Mil-Homens D, Rocha EP, Fialho AM, Baldwin A, Dowson C, Barrell BG, Govan JR, Vandamme P, Hart CA, Mahenthiralingam E, Parkhill J. The genome of Burkholderia cenocepacia J2315, an epidemic pathogen of cystic fibrosis patients. J Bacteriol. 2009;191(1):261–277. doi: 10.1128/JB.01230-08. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

