A novel computational model of the human ventricular action potential and Ca transient
- PMID: 19835882
- PMCID: PMC2813400
- DOI: 10.1016/j.yjmcc.2009.09.019
A novel computational model of the human ventricular action potential and Ca transient
Abstract
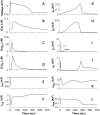
We have developed a detailed mathematical model for Ca handling and ionic currents in the human ventricular myocyte. Our aims were to: (1) simulate basic excitation-contraction coupling phenomena; (2) use realistic repolarizing K current densities; (3) reach steady-state. The model relies on the framework of the rabbit myocyte model previously developed by our group, with subsarcolemmal and junctional compartments where ion channels sense higher [Ca] vs. bulk cytosol. Ion channels and transporters have been modeled on the basis of the most recent experimental data from human ventricular myocytes. Rapidly and slowly inactivating components of I(to) have been formulated to differentiate between endocardial and epicardial myocytes. Transmural gradients of Ca handling proteins and Na pump were also simulated. The model has been validated against a wide set of experimental data including action potential duration (APD) adaptation and restitution, frequency-dependent increase in Ca transient peak and [Na](i). Interestingly, Na accumulation at fast heart rate is a major determinant of APD shortening, via outward shifts in Na pump and Na-Ca exchange currents. We investigated the effects of blocking K currents on APD and repolarization reserve: I(Ks) block does not affect the former and slightly reduces the latter; I(K1) blockade modestly increases APD and more strongly reduces repolarization reserve; I(Kr) blockers significantly prolong APD, an effect exacerbated as pacing frequency is decreased, in good agreement with experimental results in human myocytes. We conclude that this model provides a useful framework to explore excitation-contraction coupling mechanisms and repolarization abnormalities at the single myocyte level.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures







References
-
- Bondarenko VE, Szigeti GP, Bett GCL, Kim SJ, Rasmusson RL. Computer model of action potential of mouse ventricular myocytes. Am J Physiol Heart Circ Physiol. 2004;287(3):H1378–1403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

