Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3)
- PMID: 19836308
- PMCID: PMC2818223
- DOI: 10.1016/j.it.2009.09.007
Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3)
Abstract
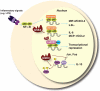
In just a few years, the view of glycogen synthase kinase-3 (GSK3) has been transformed from an obscure enzyme seldom encountered in the immune literature to one implicated in an improbably large number of roles. GSK3 is a crucial regulator of the balance between pro- and anti-inflammatory cytokine production in both the periphery and the central nervous system, so that GSK3 inhibitors such as lithium can diminish inflammation. GSK3 influences T-cell proliferation, differentiation and survival. Many effects stem from GSK3 regulation of critical transcription factors, such as NF-kappaB, NFAT and STATs. These discoveries led to the rapid application of GSK3 inhibitors to animal models of sepsis, arthritis, colitis, multiple sclerosis and others, demonstrating their potential for therapeutic intervention.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

