Adenine removal activity and bacterial complementation with the human MutY homologue (MUTYH) and Y165C, G382D, P391L and Q324R variants associated with colorectal cancer
- PMID: 19836313
- PMCID: PMC2789978
- DOI: 10.1016/j.dnarep.2009.09.009
Adenine removal activity and bacterial complementation with the human MutY homologue (MUTYH) and Y165C, G382D, P391L and Q324R variants associated with colorectal cancer
Abstract
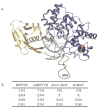
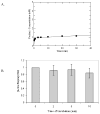
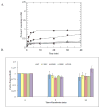
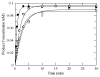
MUTYH-associated polyposis (MAP) is the only inherited colorectal cancer syndrome that is associated with inherited biallelic mutations in a base excision repair gene. The MUTYH glycosylase plays an important role in preventing mutations associated with 8-oxoguanine (OG) by removing adenine residues that have been misincorporated opposite OG. MAP-associated mutations are present throughout MUTYH, with a large number coding for missense variations. To date the available information on the functional properties of MUTYH variants is conflicting. In this study, a kinetic analysis of the adenine glycosylase activity of MUTYH and several variants was undertaken using a correction for active fraction to control for differences due to overexpression and purification. Using these methods, the rate constants for steps involved in the adenine removal process were determined for the MAP variants Y165C, G382D, P391L and Q324R MUTYH. Under single-turnover conditions, the rate of adenine removal for these four variants was found to be 30-40% of WT MUTYH. In addition, the ability of MUTYH and the variants to suppress mutations and complement for the absence of MutY in Escherichia coli was assessed using rifampicin resistance assays. The presence of WT and Q324R MUTYH resulted in complete suppression of the mutation frequency, while G382D MUTYH showed reduced ability to suppress the mutation frequency. In contrast, the mutation frequency observed upon expression of P391L and Y165C MUTYH were similar to the controls, suggesting no activity toward preventing DNA mutations. Notably, though all variations studied herein resulted in similar reductions in adenine glycosylase activity, the effects in the bacterial complementation are quite different. This suggests that the consequences of a specific amino acid variation on overall repair in a cellular context may be magnified.
Figures


References
-
- Ames BN, Gold LS. Endogenous mutagens and the causes of aging and cancer. Mutat Res. 1991;250:121. - PubMed
-
- Klaunig JE, Kamendulis LM. The Role of Oxidative Stress in Carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–267. - PubMed
-
- Neeley WL, Essigmann JM. Mechanisms of Formation, Genotoxicity, and Mutation of Guanine Oxidation Products. Chem Res Toxicol. 2006;19:491–505. - PubMed
-
- Burrows CM, Muller J. Oxidative Nucleobase Modifications Leading to Stand Scission. Chem Reviews. 1998;98:1109–1151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

