Impaired DNA damage response--an Achilles' heel sensitizing cancer to chemotherapy and radiotherapy
- PMID: 19836377
- PMCID: PMC2783472
- DOI: 10.1016/j.ejphar.2009.05.032
Impaired DNA damage response--an Achilles' heel sensitizing cancer to chemotherapy and radiotherapy
Abstract
Despite the progress in targeting particular molecular abnormalities specific to different cancers (targeted therapy), chemo- and radiotherapies are still the most effective of all anticancer modalities. Induction of DNA damage and inhibition of cell proliferation are the objects of most chemotherapeutic agents and radiation. Their effectiveness was initially thought to be due to the high rate of proliferation of cancer cells. However, normal cell proliferation rate in some tissues often exceeds that of curable tumors. Most tumors have impaired DNA damage response (DDR) and the evidence is forthcoming that this confers sensitivity to chemo- or radiotherapy. DDR is a complex set of events which elicits a plethora of molecular interactions engaging signaling pathways designed to: (a) halt cell cycle progression and division to prevent transfer of DNA damage to progeny cells; (b) increase the accessibility of the damaged sites to the DNA repair machinery; (c) engage DNA repair mechanisms and (d) activate the apoptotic pathway when DNA cannot be successfully repaired. A defective DDR makes cancer cells unable to effectively stop cell cycle progression, engage in DNA repair and/or trigger the apoptotic program when treated with DNA damaging drugs. With continued exposure to the drug, such cells accumulate DNA damage which leads to their reproductive death that may have features of cell senescence. Cancers with nonfunctional BRCA1 and BRCA2 are particularly sensitive to combined treatment with DNA damaging drugs and inhibitors of poly(ADP-ribose) polymerase. Antitumor strategies are being designed to treat cancers having particular defects in their DDR, concurrent with protecting normal cells.
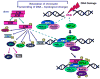
Figures

References
-
- Abraham RT, Tibbetts RS. Guiding ATM to broken DNA. Science. 2005;308:510–511. - PubMed
-
- Ahn JY, Schwartz JK, Piwnica-Worms H, Canman CE. Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res. 2000;60:5934–5936. - PubMed
-
- Ahn JY, Li X, Davis HL, Canman CE. Phosphorylation of threonine 68 promotes ologomerization and autophosphorylation of Chk2 protein kinase via the forkhead-associated domain. J Biol Chem. 2002;277:19389–19395. - PubMed
-
- Ahn J, Urist M, Prives C. The Chk2 protein kinase. DNA Repair. 2004;3:1039–1047. - PubMed
-
- Albino AP, Huang X, Yang J, Gietl D, Jorgensen E, Traganos F, Darzynkiewicz Z. Induction of histone H2AX phosphorylation in A549 human pulmonary epithelial cells by tobacco smoke and in human bronchial epithelial cells by smoke condensate: A new assay to detect the presence of potential carcinogens in tobacco. Cell Cycle. 2004;3:1062–1068. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

