Low nitric oxide synthases (NOSs) in eyes with age-related macular degeneration (AMD)
- PMID: 19836390
- PMCID: PMC2789190
- DOI: 10.1016/j.exer.2009.10.004
Low nitric oxide synthases (NOSs) in eyes with age-related macular degeneration (AMD)
Abstract
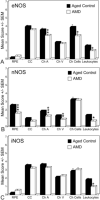
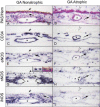
Nitric oxide (NO) production by vascular endothelium is important in regulation of blood flow. Reduced production of NO can adversely affect blood flow and other vascular functions. We investigated the expression of three nitric oxide synthase (NOS) isoforms in retina and choroid of aged human eyes and eyes with AMD. Alkaline phosphatase immunohistochemistry was performed using antibodies against inducible (iNOS), neuronal (nNOS), and endothelial (eNOS) NOSs on cryopreserved sections from aged control donor eyes (n = 13) and eyes with AMD (n = 22). CD34 antibody was used as an endothelial cell (EC) marker. Three independent masked observers scored the intensity of the immunohistochemical reaction product. Mean scores from the aged control and AMD eyes were statistically compared. In aged control retinas, nNOS was in ganglion cells (RGCs) and neurons of both nuclear layers. In choroid, perivascular nerve fibers and retinal pigment epithelial (RPE) cells were nNOS+. eNOS and iNOS were confined to the retinal and choroidal vascular ECs. Some cells presumably melanocytes or dendritic cells in choroid were also eNOS+. In AMD eyes, nNOS was significantly lower in RGCs, neurons, retinal vessels and RPE (p < or = 0.05) compared to the aged control eyes. iNOS and eNOS showed no significant differences between aged control and AMD eyes except that there was significantly less eNOS in choroidal arteries (p = 0.006) and choroidal cells (p = 0.03) of AMD eyes. Although NO was not measured directly, these findings suggest that there is less NO produced in AMD eyes. The decrease in retinal nNOS in AMD eyes is probably related to neuronal degeneration. The decrease in nNOS and eNOS in AMD choroid could be associated with vasoconstriction and hemodynamic changes.
Figures






References
-
- Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134. - PubMed
-
- Bergua A, Mayer B, Neuhuber WL. Nitrergic and VIPergic neurons in the choroid and ciliary ganglion of the duck Anis carina. Anat Embryol (Berl) 1996;193:239–248. - PubMed
-
- Bhutto IA, Kim SY, McLeod D, S, Merges CA, Fukai N, Olsen BR, Lutty GA. Localization of collagen XVIII and the endostatin portion of collagen XVIII in aged human control eyes and eyes with age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2004;45:1544–1552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

