Sweet talk in the brain: glucosensing, neural networks, and hypoglycemic counterregulation
- PMID: 19836412
- PMCID: PMC2813965
- DOI: 10.1016/j.yfrne.2009.10.006
Sweet talk in the brain: glucosensing, neural networks, and hypoglycemic counterregulation
Abstract
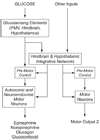
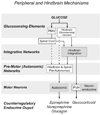
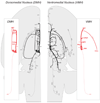
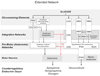
Glucose is the primary fuel for the vast majority of cells, and animals have evolved essential cellular, autonomic, endocrine, and behavioral measures to counteract both hypo- and hyperglycemia. A central component of these counterregulatory mechanisms is the ability of specific sensory elements to detect changes in blood glucose and then use that information to produce appropriate counterregulatory responses. Here we focus on the organization of the neural systems that are engaged by glucosensing mechanisms when blood glucose concentrations fall to levels that pose a physiological threat. We employ a classic sensory-motor integrative schema to describe the peripheral, hindbrain, and hypothalamic components that make up counterregulatory mechanisms in the brain. We propose that models previously developed to describe how the forebrain modulates autonomic reflex loops in the hindbrain offer a reasoned framework for explaining how counterregulatory neural mechanisms in the hypothalamus and hindbrain are structured.
2009 Elsevier Inc. All rights reserved.
Figures


References
-
- Adachi A, Shimizu N, Oomura Y, Kobáshi M. Convergence of hepatoportal glucose-sensitive afferent signals to glucose-sensitive units within the nucleus of the solitary tract. Neurosci. Letters. 1984;46:215–218. - PubMed
-
- Anand BK, Chhina GS, Sharma KN, Dua S, Singh B. Activity of single neurons in the hypothalamic feeding centers: effect of glucose. Am. J. Physiol. 1964;207:1146–1154. - PubMed
-
- Andrew SF, Dinh TT, Ritter S. Localized glucoprivation of hindbrain sites elicits corticosterone and glucagon secretion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R1792–R1798. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

