Writing memories with light-addressable reinforcement circuitry
- PMID: 19837039
- PMCID: PMC3920284
- DOI: 10.1016/j.cell.2009.08.034
Writing memories with light-addressable reinforcement circuitry
Erratum in
- Cell. 2009 Nov 25;139(5):1022
Abstract
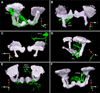
Dopaminergic neurons are thought to drive learning by signaling changes in the expectations of salient events, such as rewards or punishments. Olfactory conditioning in Drosophila requires direct dopamine action on intrinsic mushroom body neurons, the likely storage sites of olfactory memories. Neither the cellular sources of the conditioning dopamine nor its precise postsynaptic targets are known. By optically controlling genetically circumscribed subsets of dopaminergic neurons in the behaving fly, we have mapped the origin of aversive reinforcement signals to the PPL1 cluster of 12 dopaminergic cells. PPL1 projections target restricted domains in the vertical lobes and heel of the mushroom body. Artificially evoked activity in a small number of identifiable cells thus suffices for programming behaviorally meaningful memories. The delineation of core reinforcement circuitry is an essential first step in dissecting the neural mechanisms that compute and represent valuations, store associations, and guide actions.
Figures

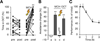


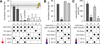

Comment in
-
New routes for memory retrieval and reinforcement.Cell. 2009 Oct 16;139(2):225-7. doi: 10.1016/j.cell.2009.09.031. Cell. 2009. PMID: 19837025
References
-
- Berg HC. Ecoli in motion. New York: Springer; 2004.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

