Extracellular diffusion and permeability effects on NO-RBCs interactions using an experimental and theoretical model
- PMID: 19837099
- PMCID: PMC2813360
- DOI: 10.1016/j.mvr.2009.10.002
Extracellular diffusion and permeability effects on NO-RBCs interactions using an experimental and theoretical model
Abstract
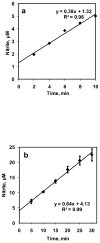
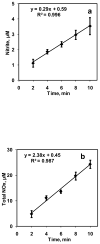
Nitric oxide (NO) is a potent vasodilator and its homeostasis depends on interaction with RBCs. A key factor in understanding NO-RBC interactions in vascular lumen is a comprehensive analysis of product identification and quantification. In this context, administration of NO during in vitro NO-RBC interactions becomes a crucial variable. In this study, we designed a bioreactor that maintains a precise NO concentration in the headspace that diffuses to RBCs suspension to study the quantitative effect of NO concentration and hematocrit (Hct) on NO-RBC interactions. The products of NO-RBC reaction (nitrite and total nitrogen species (total NOx)) were measured by chemiluminescence assay. A mathematical model simulating NO biotransport to a single RBC was developed to (1) estimate NO-RBC reaction rate constant; (2) predict the NO concentrations in the bulk RBC suspension and at the RBC membrane for RBC membrane NO permeability (P(m)) values of 0.0415-40 cm/s. Measured nitrite and total NOx concentrations increased with increase in headspace NO concentration whereas nitrite concentrations decreased with hematocrit and total NOx concentrations increased with hematocrit. This indicates that the extracellular resistance is a controlling factor for RBC uptake of NO. Modeling results showed that the effective reaction rate constant (k(eff)) for NO-RBC interactions was 2.32 x 10(4)-1.08 x 10(6) M(-1) s(-1). Results also predict that the membrane permeability in the range of 0.0415-0.4 cm/s is required to maintain physiologically relevant levels of NO at the smooth muscle cell layer. The effective reaction rate constant increased with increase in P(m) and magnitude of increase was higher at 45% Hct. For all P(m) values, the k(hb)/k(eff) ratios were lower for 45% Hct as compared to 5% Hct indicating extracellular resistance is important for RBC NO uptake. Our experimental and mathematical analyses of NO-RBC interactions indicate that both unstirred layer and RBC membrane have a significant effect on NO transport to RBCs. In addition, the membrane permeability in the range of 0.0415-0.4 cm/s is required to maintain sufficient NO concentrations at the smooth muscle cell layer.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures







References
-
- Azarov I, et al. Nitric oxide scavenging by red blood cells as a function of hematocrit and oxygenation. J Biol Chem 2005 - PubMed
-
- Buerk DG, et al. Modeling the influence of superoxide dismutase on superoxide and nitric oxide interactions, including reversible inhibition of oxygen consumption. Free Radic Biol Med. 2003;34:1488–503. - PubMed
-
- Butler AR, et al. Diffusion of nitric oxide and scavenging by blood in the vasculature. Biochim Biophys Acta. 1998;1425:168–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

