Tissue-specific activities of an immune signaling module regulate physiological responses to pathogenic and nutritional bacteria in C. elegans
- PMID: 19837372
- PMCID: PMC2772662
- DOI: 10.1016/j.chom.2009.09.001
Tissue-specific activities of an immune signaling module regulate physiological responses to pathogenic and nutritional bacteria in C. elegans
Abstract
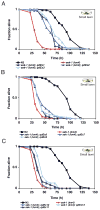
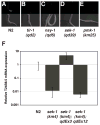
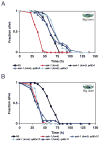
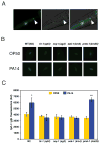
Microbes represent both an essential source of nutrition and a potential source of lethal infection to the nematode Caenorhabditis elegans. Immunity in C. elegans requires a signaling module comprised of orthologs of the mammalian Toll-interleukin-1 receptor (TIR) domain protein SARM, the mitogen-activated protein kinase kinase kinase (MAPKKK) ASK1, and MAPKK MKK3, which activates p38 MAPK. We determined that the SARM-ASK1-MKK3 module has dual tissue-specific roles in the C. elegans response to pathogens--in the cell-autonomous regulation of innate immunity and the neuroendocrine regulation of serotonin-dependent aversive behavior. SARM-ASK1-MKK3 signaling in the sensory nervous system also regulates egg-laying behavior that is dependent on bacteria provided as a nutrient source. Our data demonstrate that these physiological responses to bacteria share a common mechanism of signaling through the SARM-ASK1-MKK3 module and suggest the co-option of ancestral immune signaling pathways in the evolution of physiological responses to microbial pathogens and nutrients.
Figures


References
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

