Mechanism of inhibition of HIV-1 reverse transcriptase by 4'-Ethynyl-2-fluoro-2'-deoxyadenosine triphosphate, a translocation-defective reverse transcriptase inhibitor
- PMID: 19837673
- PMCID: PMC2790999
- DOI: 10.1074/jbc.M109.036616
Mechanism of inhibition of HIV-1 reverse transcriptase by 4'-Ethynyl-2-fluoro-2'-deoxyadenosine triphosphate, a translocation-defective reverse transcriptase inhibitor
Abstract
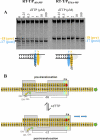
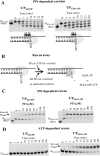
Nucleoside reverse transcriptase inhibitors (NRTIs) are employed in first line therapies for the treatment of human immunodeficiency virus (HIV) infection. They generally lack a 3'-hydroxyl group, and thus when incorporated into the nascent DNA they prevent further elongation. In this report we show that 4'-ethynyl-2-fluoro-2'-deoxyadenosine (EFdA), a nucleoside analog that retains a 3'-hydroxyl moiety, inhibited HIV-1 replication in activated peripheral blood mononuclear cells with an EC(50) of 0.05 nm, a potency several orders of magnitude better than any of the current clinically used NRTIs. This exceptional antiviral activity stems in part from a mechanism of action that is different from approved NRTIs. Reverse transcriptase (RT) can use EFdA-5'-triphosphate (EFdA-TP) as a substrate more efficiently than the natural substrate, dATP. Importantly, despite the presence of a 3'-hydroxyl, the incorporated EFdA monophosphate (EFdA-MP) acted mainly as a de facto terminator of further RT-catalyzed DNA synthesis because of the difficulty of RT translocation on the nucleic acid primer possessing 3'-terminal EFdA-MP. EFdA-TP is thus a translocation-defective RT inhibitor (TDRTI). This diminished translocation kept the primer 3'-terminal EFdA-MP ideally located to undergo phosphorolytic excision. However, net phosphorolysis was not substantially increased, because of the apparently facile reincorporation of the newly excised EFdA-TP. Our molecular modeling studies suggest that the 4'-ethynyl fits into a hydrophobic pocket defined by RT residues Ala-114, Tyr-115, Phe-160, and Met-184 and the aliphatic chain of Asp-185. These interactions, which contribute to both enhanced RT utilization of EFdA-TP and difficulty in the translocation of 3'-terminal EFdA-MP primers, underlie the mechanism of action of this potent antiviral nucleoside.
Figures



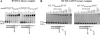

References
-
- Hammer S. M., Saag M. S., Schechter M., Montaner J. S., Schooley R. T., Jacobsen D. M., Thompson M. A., Carpenter C. C., Fischl M. A., Gazzard B. G., Gatell J. M., Hirsch M. S., Katzenstein D. A., Richman D. D., Vella S., Yeni P. G., Volberding P. A. (2006) Top. HIV Med. 14, 827–843 - PubMed
-
- Parniak M. A., Sluis-Cremer N. (2000) Adv. Pharmacol. 49, 67–109 - PubMed
-
- De Clercq E. (2007) Verh. K. Acad. Geneeskd. Belg. 69, 81–104 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

