Preclinical properties of 18F-AV-45: a PET agent for Abeta plaques in the brain
- PMID: 19837759
- PMCID: PMC3065020
- DOI: 10.2967/jnumed.109.065284
Preclinical properties of 18F-AV-45: a PET agent for Abeta plaques in the brain
Abstract
beta-amyloid plaques (Abeta plaques) in the brain, containing predominantly fibrillary Abeta peptide aggregates, represent a defining pathologic feature of Alzheimer disease (AD). Imaging agents targeting the Abeta plaques in the living human brain are potentially valuable as biomarkers of pathogenesis processes in AD. (E)-4-(2-(6-(2-(2-(2-(18)F-fluoroethoxy)ethoxy)ethoxy)pyridin-3-yl)vinyl)-N-methyl benzenamine ((18)F-AV-45) is such as an agent currently in phase III clinical studies for PET of Abeta plaques in the brain.
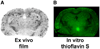
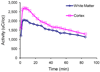
Methods: In vitro binding of (18)F-AV-45 to Abeta plaques in the postmortem AD brain tissue was evaluated by in vitro binding assay and autoradiography. In vivo biodistribution of (18)F-AV-45 in mice and ex vivo autoradiography of AD transgenic mice (APPswe/PSEN1) with Abeta aggregates in the brain were performed. Small-animal PET of a monkey brain after an intravenous injection of (18)F-AV-45 was evaluated.
Results: (18)F-AV-45 displayed a high binding affinity and specificity to Abeta plaques (K(d), 3.72 +/- 0.30 nM). In vitro autoradiography of postmortem human brain sections showed substantial plaque labeling in AD brains and not in the control brains. Initial high brain uptake and rapid washout from the brain of healthy mice and monkey were observed. Metabolites produced in the blood of healthy mice after an intravenous injection were identified. (18)F-AV-45 displayed excellent binding affinity to Abeta plaques in the AD brain by ex vivo autoradiography in transgenic AD model mice. The results lend support that (18)F-AV-45 may be a useful PET agent for detecting Abeta plaques in the living human brain.
Figures





References
-
- Hardy J. Has the amyloid cascade hypothesis for Alzheimer’s disease been proved? Curr Alzheimer Res. 2006;3:71–73. - PubMed
-
- Minoshima S. Imaging Alzheimer’s disease: clinical applications. Neuroimaging Clin N Am. 2003;13:769–780. - PubMed
-
- Silverman DH. Brain 18F-FDG PET in the diagnosis of neurodegenerative dementias: comparison with perfusion SPECT and with clinical evaluations lacking nuclear imaging. J Nucl Med. 2004;45:594–607. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
