Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial
- PMID: 19837791
- PMCID: PMC2797966
- DOI: 10.2337/dc09-1502
Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial
Abstract
Objective: To determine whether continuous glucose monitoring (CGM) is effective in the management of type 1 diabetes when implemented in a manner that more closely approximates clinical practice.
Research design and methods: After completion of a 6-month randomized controlled trial (RCT) evaluating CGM in children, adolescents, and adults with type 1 diabetes, CGM was initiated in the trial's control group with less intensive training and follow-up than was included in the RCT. Subjects had an outpatient training session, two follow-up phone calls, and outpatient visits at 1, 4, 13, and 26 weeks. For subjects with baseline A1C > or =7.0%, the primary outcome was change in A1C at 6 months.
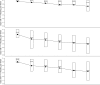
Results: CGM use decreased from a median of 7.0 days/week in the first month in the > or =25-year-old group, 6.3 days/week in the 15-24 year olds, and 6.8 days/week in the 8-14 year olds to 6.5, 3.3, and 3.7 days/week in the 6th month, respectively (P < 0.001 for each age-group). Among subjects with baseline A1C > or =7.0%, CGM use was associated with A1C reduction after 6 months (P = 0.02 adjusted for age-group). Severe hypoglycemia decreased from 27.7 events per 100 person-years in the 6-month control phase of the RCT to 15.0 events per 100 person-years in the 6-month follow-up CGM phase (P = 0.08).
Conclusions: Frequent use of CGM in a clinical care setting may improve A1C and reduce episodes of hypoglycemia. However, sustained frequent use of CGM is less likely in children and adolescents than in adults.
Trial registration: ClinicalTrials.gov NCT00406133.
Figures
References
-
- Resnick HE, Foster GL, Bardsley J, Ratner RE: Achievement of American Diabetes Association clinical practice recommendations among U.S. adults with diabetes, 1999–2002: the National Health and Nutrition Examination Survey. Diabetes Care 2006; 29: 531– 537 - PubMed
-
- Bode BW, Schwartz S, Stubbs HA, Block JE: Glycemic characteristics in continuously monitored patients with type 1 and type 2 diabetes: normative values. Diabetes Care 2005; 28: 2361– 2366 - PubMed
-
- Boland E, Monsod T, Delucia M, Brandt CA, Fernando S, Tamborlane WV: Limitations of conventional methods of self-monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with type 1 diabetes. Diabetes Care 2001; 24: 1858– 1862 - PubMed
-
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008; 359: 1464– 1476 - PubMed

