Brain-endocrine interactions: a microvascular route in the mediobasal hypothalamus
- PMID: 19837874
- PMCID: PMC2819742
- DOI: 10.1210/en.2009-0584
Brain-endocrine interactions: a microvascular route in the mediobasal hypothalamus
Abstract
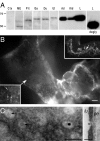
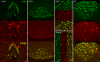
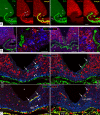
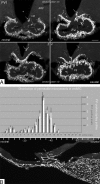
Blood-borne hormones acting in the mediobasal hypothalamus, like those controlling food intake, require relatively direct access to target chemosensory neurons of the arcuate nucleus (ARC). An anatomical substrate for this is a permeable microvasculature with fenestrated endothelial cells in the ARC, a system that has awaited comprehensive documentation. Here, the immunofluorescent detection of endothelial fenestral diaphragms in the rat ARC allowed us to quantitate permeable microvessels throughout its rostrocaudal extent. We have determined that permeable microvessels are part of the subependymal plexus irrigating exclusively the ventromedial (vm) ARC from the subadjacent neuroendocrine median eminence. Unexpectedly, permeable microvessels were concentrated proximal to the pituitary stalk. This marked topography strongly supports the functional importance of retrograde blood flow from the pituitary to the vmARC, therefore making a functional relationship between peripheral long-loop, pituitary short-loop, and neuroendocrine ultra-short loop feedback, altogether converging for integration in the vmARC (formerly known as the hypophysiotrophic area), thereby so pivotal as a multicompetent brain endocrinostat.
Figures


References
-
- Grove KL, Grayson BE, Glavas MM, Xiao XQ, Smith MS 2005 Development of metabolic systems. Physiol Behav 86:646–660 - PubMed
-
- Cone RD 2006 Studies on the physiological functions of the melanocortin system. Endocr Rev 27:736–749 - PubMed
-
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW 2006 Central nervous system control of food intake and body weight. Nature 443:289–295 - PubMed
-
- Badman MK, Flier JS 2007 The adipocyte as an active participant in energy balance and metabolism. Gastroenterology 132:2103–2115 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

