Barnacle cement: a polymerization model based on evolutionary concepts
- PMID: 19837892
- PMCID: PMC2762877
- DOI: 10.1242/jeb.029884
Barnacle cement: a polymerization model based on evolutionary concepts
Abstract
Enzymes and biochemical mechanisms essential to survival are under extreme selective pressure and are highly conserved through evolutionary time. We applied this evolutionary concept to barnacle cement polymerization, a process critical to barnacle fitness that involves aggregation and cross-linking of proteins. The biochemical mechanisms of cement polymerization remain largely unknown. We hypothesized that this process is biochemically similar to blood clotting, a critical physiological response that is also based on aggregation and cross-linking of proteins. Like key elements of vertebrate and invertebrate blood clotting, barnacle cement polymerization was shown to involve proteolytic activation of enzymes and structural precursors, transglutaminase cross-linking and assembly of fibrous proteins. Proteolytic activation of structural proteins maximizes the potential for bonding interactions with other proteins and with the surface. Transglutaminase cross-linking reinforces cement integrity. Remarkably, epitopes and sequences homologous to bovine trypsin and human transglutaminase were identified in barnacle cement with tandem mass spectrometry and/or western blotting. Akin to blood clotting, the peptides generated during proteolytic activation functioned as signal molecules, linking a molecular level event (protein aggregation) to a behavioral response (barnacle larval settlement). Our results draw attention to a highly conserved protein polymerization mechanism and shed light on a long-standing biochemical puzzle. We suggest that barnacle cement polymerization is a specialized form of wound healing. The polymerization mechanism common between barnacle cement and blood may be a theme for many marine animal glues.
Figures


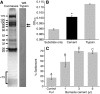
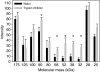

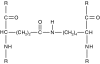
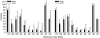
References
-
- Barlow, D. E., Dickinson, G. H., Orihuela, B., Rittschof, D. and Wahl, K. J. (2009). In situ ATR-FTIR characterization of primary cement interfaces of the barnacle Balanus amphitrite. Biofouling 25, 359-366. - PubMed
-
- Barnes, H. and Blackstock, J. (1976). Further observations on the biochemical composition of the cement of Lepas fasicularis Ellis & Solander; electrophoretic examination of the protein moieties under various conditions. J. Exp. Mar. Biol. Ecol. 25, 263-271.
-
- Bauchau, A. G. (1981). Crustaceans. In Invertebrate Blood Cells, vol. 2 (ed. N. A. Ratcliff and A. F. Rowley), pp. 385-420. London: Academic Press.
-
- Biro, A., Herincs, Z., Fellinger, E., Szilagyi, L., Barad, Z., Gergely, J., Graf, L. and Sarmay, G. (2003). Characterization of a trypsin-like serine protease of activated B cells mediating the cleavage of surface proteins. Biochim. Biophys. Acta 1624, 60-69. - PubMed
-
- Bradford, M. M. (1976). Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 72, 248-254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

