Defective O-glycosylation due to a novel homozygous S129P mutation is associated with lack of fibroblast growth factor 23 secretion and tumoral calcinosis
- PMID: 19837926
- PMCID: PMC2775647
- DOI: 10.1210/jc.2009-0961
Defective O-glycosylation due to a novel homozygous S129P mutation is associated with lack of fibroblast growth factor 23 secretion and tumoral calcinosis
Abstract
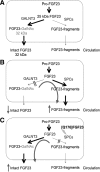
Background: Homozygous mutations in fibroblast growth factor (FGF23) have recently been described as the genetic cause of one form of hyperphosphatemic tumoral calcinosis (HFTC). However, it remained unclear to date how these mutations lead to loss of biologically active FGF23 in the circulation.
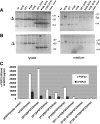
Methods: We here report a novel homozygous mutation, c.385T>C in FGF23 exon 2, which changes codon 129 from serine to proline (S129P) in a previously described individual affected by HFTC. The S129P mutation as well as two known FGF23 mutations, S71G and S129F, were introduced into an expression vector encoding wild-type (wt) human (h) FGF23 to yield [P129]hFGF23, [F129]hFGF23, and [G71]hFGF23; whole lysates, glycoprotein fractions, and conditioned media from HEK293 and COS-7 cells expressing these constructs were subjected to Western blot analysis using affinity-purified goat anti-hFGF23(51-69) and anti-hFGF23(206-222) antibodies.
Results: We detected 25- and 32-kDa protein species in total lysates of HEK293 cells expressing wt-hFGF23. The 32-kDa band, representing O-glycosylated hFGF23, was not detectable in the glycoprotein fraction of lysates from HEK293 cells expressing [P129]hFGF23, and in comparison with wt-FGF23 only small amounts of [P129]hFGF23 were secreted into the medium. Similar results were obtained for cells expressing [G71]hFGF23 and [F129]hFGF23.
Conclusion: Our data for the first time directly show that FGF23 mutations associated with HFTC impair O-glycosylation in vitro resulting in poor secretion of the mutant hormone thereby explaining the characteristic hyperphosphatemic phenotype of homozygous carriers in vivo.
Figures



References
-
- Giard JM 1998 Sur la calcifcation hibernale. Compes Rend Seanes Soc Biol So 34:1013–1015
-
- Duret MH 1899 Tumeurs multiples et singulieres des bourses sereuses. Bull Mem Soc Ant Paris 74:725–731
-
- Eddy MC, Jan De Beur SM, Yandow SM, McAlister WH, Shore EM, Kaplan FS, Whyte MP, Levine MA 2000 Deficiency of the α-subunit of the stimulatory G protein and severe extraskeletal ossification. J Bone Miner Res 15:2074–2083 - PubMed
-
- Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M, Khamaysi Z, Behar D, Petronius D, Friedman V, Zelikovic I, Raimer S, Metzker A, Richard G, Sprecher E 2004 Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet 36:579–581 - PubMed
-
- Kato K, Jeanneau C, Tarp MA, Benet-Pagès A, Lorenz-Depiereux B, Bennett EP, Mandel U, Strom TM, Clausen H 2006 Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem 281:18370–18377 - PubMed

