The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination
- PMID: 19838178
- PMCID: PMC2783566
- DOI: 10.1038/nn.2410
The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination
Abstract
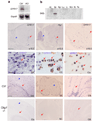
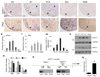
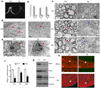
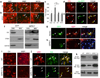
The basic helix-loop-helix transcription factor Olig1 promotes oligodendrocyte maturation and is required for myelin repair. We characterized an Olig1-regulated G protein-coupled receptor, GPR17, whose function is to oppose the action of Olig1. Gpr17 was restricted to oligodendrocyte lineage cells, but was downregulated during the peak period of myelination and in adulthood. Transgenic mice with sustained Gpr17 expression in oligodendrocytes exhibited stereotypic features of myelinating disorders in the CNS. Gpr17 overexpression inhibited oligodendrocyte differentiation and maturation both in vivo and in vitro. Conversely, Gpr17 knockout mice showed early onset of oligodendrocyte myelination. The opposing action of Gpr17 on oligodendrocyte maturation reflects, at least partially, upregulation and nuclear translocation of the potent oligodendrocyte differentiation inhibitors ID2/4. Collectively, these findings suggest that GPR17 orchestrates the transition between immature and myelinating oligodendrocytes via an ID protein-mediated negative regulation and may serve as a potential therapeutic target for CNS myelin repair.
Figures

References
-
- Trapp BD, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. - PubMed
-
- Berger J, Moser HW, Forss-Petter S. Leukodystrophies: recent developments in genetics, molecular biology, pathogenesis and treatment. Curr Opin Neurol. 2001;14:305–312. - PubMed
-
- Pfeiffer SE, Warrington AE, Bansal r. The oligodendrocyte and its many cellular processes. Trends in Cell Biology. 1993;3:191–197. - PubMed
-
- Wang S, Sdrulla A, Johnson JE, Yokota Y, Barres BA. A role for the helix-loop-helix protein Id2 in the control of oligodendrocyte development. Neuron. 2001;29:603–614. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

