Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells
- PMID: 19838182
- PMCID: PMC2833279
- DOI: 10.1038/nmat2563
Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells
Abstract
Growing evidence suggests that physical microenvironments and mechanical stresses, in addition to soluble factors, help direct mesenchymal-stem-cell fate. However, biological responses to a local force in embryonic stem cells remain elusive. Here we show that a local cyclic stress through focal adhesions induced spreading in mouse embryonic stem cells but not in mouse embryonic stem-cell-differentiated cells, which were ten times stiffer. This response was dictated by the cell material property (cell softness), suggesting that a threshold cell deformation is the key setpoint for triggering spreading responses. Traction quantification and pharmacological or shRNA intervention revealed that myosin II contractility, F-actin, Src or cdc42 were essential in the spreading response. The applied stress led to oct3/4 gene downregulation in mES cells. Our findings demonstrate that cell softness dictates cellular sensitivity to force, suggesting that local small forces might have far more important roles in early development of soft embryos than previously appreciated.
Figures

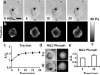
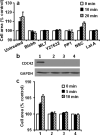
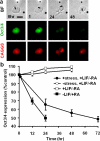
Comment in
-
Cell rheology: Stressed-out stem cells.Nat Mater. 2010 Jan;9(1):4-6. doi: 10.1038/nmat2589. Nat Mater. 2010. PMID: 20019660 No abstract available.
References
-
- Daley GQ, Scadden DT. Prospects for stem cell-based therapy. Cell. 2008;132:544–548. - PubMed
-
- Dimos JT, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. - PubMed
-
- Park IH, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

