Determination of preferred pH for root-knot nematode aggregation using pluronic F-127 gel
- PMID: 19838866
- PMCID: PMC2780626
- DOI: 10.1007/s10886-009-9703-8
Determination of preferred pH for root-knot nematode aggregation using pluronic F-127 gel
Abstract
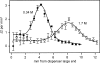
Root-knot nematodes (Meloidogyne spp.) are obligate endoparasites of a wide range of plant species. The infective stage is attracted strongly to and enters host roots at the zone of elongation, but the compounds responsible for this attraction have not been identified. We developed a simple assay to investigate nematode response to chemical gradients that uses Pluronic F-127, a synthetic block copolymer that, as a 23% aqueous solution, forms a liquid at low temperature and a gel at room temperature. Test chemicals are put into a modified pipette tip, or 'chemical dispenser,' and dispensers are inserted into the gel in which nematodes have been dispersed. Meloidogyne hapla is attracted to pH gradients formed by acetic acid and several other Brønsted acids and aggregates between pH 4.5 and 5.4. While this pH range was attractive to all tested root-knot nematode strains and species, the level of aggregation depended on the species/strain assessed. For actively growing roots, the pH at the root surface is most acidic at the zone of elongation. This observation is consistent with the idea that low pH is an attractant for nematodes. Root-knot nematodes have been reported to be attracted to carbon dioxide, but our experiments suggest that the observed attraction may be due to acidification of solutions by dissolved CO(2) rather than to CO(2) itself. These results suggest that Pluronic F-127 gel will be broadly applicable for examining responses of a range of organisms to chemical gradients or to each other.
Figures
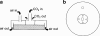



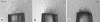

References
-
- Abad P, Gouzy J, Aury JM, Castagnone-Sereno P, Danchin EG, Deleury E, Perfus-Barbeoch L, Anthouard V, Artiguenave F, Blok VC, Caillaud MC, Coutinho PM, Dasilva C, De Luca F, Deau F, Esquibet M, Flutre T, Goldstone JV, Hamamouch N, Hewezi T, Jaillon O, Jubin C, Leonetti P, Magliano M, Maier TR, Markov GV, McVeigh P, Pesole G, Poulain J, Robinson-Rechavi M, Sallet E, Segurens B, Steinbach D, Tytgat T, Ugarte E, van Ghelder C, Veronico P, Baum TJ, Blaxter M, Bleve-Zacheo T, Davis EL, Ewbank JJ, Favery B, Grenier E, Henrissat B, Jones JT, Laudet V, Maule AG, Quesneville H, Rosso MN, Schiex T, Smant G, Weissenbach J, Wincker P. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nature Biotechnol. 2008;8:909–915. doi: 10.1038/nbt.1482. - DOI - PubMed
-
- Bird AF. Additional notes on the attractiveness of roots to plant parasitic nematodes. Nematologica. 1960;5:217.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

