The human asparaginase-like protein 1 hASRGL1 is an Ntn hydrolase with beta-aspartyl peptidase activity
- PMID: 19839645
- PMCID: PMC2782781
- DOI: 10.1021/bi901397h
The human asparaginase-like protein 1 hASRGL1 is an Ntn hydrolase with beta-aspartyl peptidase activity
Abstract
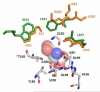
Herein we report the bacterial expression, purification, and enzymatic characterization of the human asparaginase-like protein 1 (hASRGL1). We present evidence that hASRGL1 exhibits beta-aspartyl peptidase activity consistent with enzymes designated as plant-type asparaginases, which had thus far been found in only plants and bacteria. Similar to nonmammalian plant-type asparaginases, hASRGL1 is shown to be an Ntn hydrolase for which Thr168 serves as the essential N-terminal nucleophile for intramolecular processing and catalysis, corroborated in part by abolishment of both activities through the Thr168Ala point mutation. In light of the activity profile reported here, ASRGL1s may act synergistically with protein l-isoaspartyl methyl transferase to relieve accumulation of potentially toxic isoaspartyl peptides in mammalian brain and other tissues.
Figures




References
-
- Wade HE, Elsworth R, Herbert D, Keppie J, Sargeant K. A new Lasparaginase with antitumour activity? Lancet. 1968;2:776–777. - PubMed
-
- Distasio JA, Niederman RA, Kafkewitz D, Goodman D. Purification and characterization of L-asparaginase with anti-lymphoma activity from Vibrio succinogenes. J Biol Chem. 1976;251:6929–6933. - PubMed
-
- Michalska K, Jaskolski M. Structural aspects of L-asparaginases, their friends and relations. Acta Biochim Pol. 2006;53:627–640. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

