Conservation of telomere protein complexes: shuffling through evolution
- PMID: 19839711
- PMCID: PMC2788028
- DOI: 10.3109/10409230903307329
Conservation of telomere protein complexes: shuffling through evolution
Abstract
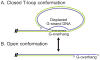
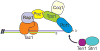
The rapid evolution of telomere proteins has hindered identification of orthologs from diverse species and created the impression that certain groups of eukaryotes have largely non-overlapping sets of telomere proteins. However, the recent identification of additional telomere proteins from various model organisms has dispelled this notion by expanding our understanding of the composition, architecture and range of telomere protein complexes present in individual species. It is now apparent that versions of the budding yeast CST complex and mammalian shelterin are present in multiple phyla. While the precise subunit composition and architecture of these complexes vary between species, the general function is often conserved. Despite the overall conservation of telomere protein complexes, there is still considerable species-specific variation, with some organisms having lost a particular subunit or even an entire complex. In some cases, complex components appear to have migrated between the telomere and the telomerase RNP. Finally, gene duplication has created telomere protein paralogs with novel functions. While one paralog may be part of a conserved telomere protein complex and have the expected function, the other paralog may serve in a completely different aspect of telomere biology.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Figures
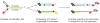



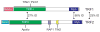
References
-
- Amiard S, Doudeau M, Pinte S, Poulet A, Lenain C, Faivre-Moskalenko C, Angelov D, Hug N, Vindigni A, Bouvet P, et al. A topological mechanism for TRF2-enhanced strand invasion. Nat Struct Mol Biol. 2007;14:147–154. - PubMed
-
- Azzalin CM, Reichenbach P, Khoriauli L, Giulotto E, Lingner J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science. 2007;318:798–801. - PubMed
-
- Baumann P, Cech TR. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science. 2001;292:1171–1175. - PubMed
-
- Bianchi A, Negrini S, Shore D. Delivery of yeast telomerase to a DNA break depends on the recruitment functions of Cdc13 and Est1. Mol Cell. 2004;16:139–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
