Vessel formation is induced prior to the appearance of cartilage in BMP-2-mediated heterotopic ossification
- PMID: 19839764
- PMCID: PMC3153372
- DOI: 10.1359/jbmr.091031
Vessel formation is induced prior to the appearance of cartilage in BMP-2-mediated heterotopic ossification
Abstract
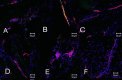
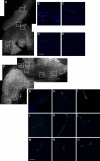
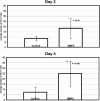
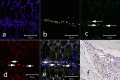
Heterotopic ossification (HO), or endochondral bone formation at nonskeletal sites, often results from traumatic injury and can lead to devastating consequences. Alternatively, the ability to harness this phenomenon would greatly enhance current orthopedic tools for treating segmental bone defects. Thus, understanding the earliest events in this process potentially would allow us to design more targeted therapies to either block or enhance this process. Using a murine model of HO induced by delivery of adenovirus-transduced cells expressing bone morphogenetic protein 2 (BMP-2), we show here that one of the earliest stages in this process is the establishment of new vessels prior to the appearance of cartilage. As early as 48 hours after induction of HO, we observed the appearance of brown adipocytes expressing vascular endothelial growth factors (VEGFs) simultaneous with endothelial progenitor replication. This was determined by using a murine model that possesses the VEGF receptor 2 (Flk1) promoter containing an endothelial cell enhancer driving the expression of nuclear-localized yellow fluorescent protein (YFP). Expression of this marker has been shown previously to correlate with the establishment of new vasculature, and the nuclear localization of YFP expression allowed us to quantify changes in endothelial cell numbers. We found a significant increase in Flk1-H2B::YFP cells in BMP-2-treated animals compared with controls. The increase in endothelial progenitors occurred 3 days prior to the appearance of early cartilage. The data collectively suggest that vascular remodeling and growth may be essential to modify the microenvironment and enable engraftment of the necessary progenitors to form endochondral bone.
(c) 2010 American Society for Bone and Mineral Research.
Figures


References
-
- Shafer J, Davis AR, Gannon FH, et al. Oxygen tension directs chondrogenic differentiation of myelo-monocytic progenitors during endochondral bone formation. Tissue Eng. 2007;13:2011–2019. - PubMed
-
- Colnot C, Lu C, Hu D, Helms JA. Distinguishing the contributions of the perichondrium, cartilage, and vascular endothelium to skeletal development. Dev Biol. 2004;269:55–69. - PubMed
-
- Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

