Antibody quantity versus quality after influenza vaccination
- PMID: 19840673
- PMCID: PMC2765411
- DOI: 10.1016/j.vaccine.2009.06.090
Antibody quantity versus quality after influenza vaccination
Abstract
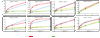
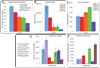
The correlates for protection against influenza infection are incompletely characterized. We have applied an ELISA strategy that distinguishes antibodies against native viral surface antigens (potentially neutralizing) from antibodies directed against internal and denatured viral proteins (not neutralizing) to three groups of vaccinated subjects: (1) participants in a study of repeated annual vaccination, (2) elderly subjects and (3) patients with Systemic Lupus Erythematosus compared to control subjects. Antibody increase after vaccination was inversely related to the level of pre-existing antibodies in all groups; most subjects had significant initial antibody levels and showed little increase in amount of antibody after vaccination, but the avidity of their serum antibodies tended to increase. Antibodies against denatured virus proteins varied with vaccine formulation; vaccines that are more recent have less total protein for the same amount of native hemagglutinin. We propose an index consisting of rank order of antibody level plus antibody avidity, both measured against native virus, plus hemagglutination-inhibition antibody titer, as a useful measure of immunity against influenza.
Figures

References
-
- Churchill ME, Stura EA, Pinilla C, Appel JR, Houghten RA, Kono DH, et al. Crystal structure of a peptide complex of anti-influenza peptide antibody Fab 26/9. Comparison of two different antibodies bound to the same peptide antigen. J Mol Biol. 1994 Aug 26;241(4):534–556. - PubMed
-
- Beyer WE, Palache AM, Sprenger MJ, Hendriksen E, Tukker JJ, Darioli R, et al. Effects of repeated annual influenza vaccination on vaccine sero-response in young and elderly adults. Vaccine. 1996 Oct;14(14):1331–1339. - PubMed
-
- Keitel WA, Cate TR, Couch RB, Huggins LL, Hess KR. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine. 1997;15(10):1114–1122. - PubMed
-
- Keitel WA, Cate TR, Couch RB. Efficacy of sequential annual vaccination with inactivated influenza virus vaccine. Am J Epidemiol. 1988;127(2):353–364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

