Clinical relevance of extent of extreme drug resistance in epithelial ovarian carcinoma
- PMID: 19840886
- PMCID: PMC4162425
- DOI: 10.1016/j.ygyno.2009.09.018
Clinical relevance of extent of extreme drug resistance in epithelial ovarian carcinoma
Abstract
Objective: The objective of this study was to evaluate the clinical significance of the extent of extreme drug resistance (EDR) in in vitro drug resistance assays in advanced epithelial ovarian, fallopian, and primary peritoneal cancers.
Methods: A retrospective study was conducted using the database for in vitro drug resistance assay (EDR Assay, Oncotech, Inc.) results for advanced stage ovarian cancer samples obtained at primary surgery between 1995 and 2009. In vitro drug resistance assay results were evaluated for thirteen drugs according to the following two groups: platinum and taxane (primary treatment group) vs remaining agents (secondary treatment group). Dual-resistance was then defined as at least one EDR in the primary and secondary treatment groups. Chemotherapy response and survival outcome were correlated with assay results.
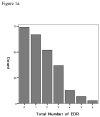
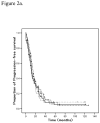
Results: There were 253 cases identified. Dual-resistance (n=53, 20.9%) was not associated with chemotherapy response (p=0.62) or survival outcomes (PFS, p=0.52; OS, p=0.11). Only one (0.4%) case exhibited complete EDR to all tested drugs, and 74 (29.4%) cases showed no EDR. There was no statistical correlation between total number of drugs in the EDR range and chemotherapy response (p=0.55), progression-free survival (PFS) (p=0.18), and overall survival (OS) (p=0.87). Proportion of EDR, defined as the ratio of the number of EDR drugs divided by all drugs for an individual patient, was also not related to chemotherapy response (p=0.37), PFS (p=0.13), or OS (p=0.13).
Conclusions: Presence of extreme drug resistance to multiple agents in the in vitro drug resistance assays was not associated with survival outcomes in advanced stage epithelial ovarian, fallopian, and primary peritoneal cancers.
Figures


References
-
- Samson DJ, Seidenfeld J, Ziegler K, Aronson N. Chemotherapy sensitivity and resistance assays: a systematic review. J Clin Oncol. 2004;22:3618–30. - PubMed
-
- Kern DH, Weisenthal LM. Highly specific prediction of antineoplastic drug resistance with an in vitro assay using suprapharmacologic drug exposures. J Natl Cancer Inst. 1990;82:582–8. - PubMed
-
- Holloway RW, Mehta RS, Finkler NJ, Li KT, McLaren CE, Parker RJ, Fruehauf JP. Association between in vitro platinum resistance in the EDR assay and clinical outcomes for ovarian cancer patients. Gynecol Oncol. 2002;87:8–16. - PubMed
-
- Matsuo K, Bond VK, Eno ML, Im DD, Rosenshein NB. Low drug resistance to both platinum and taxane chemotherapy on an in-vitro drug resistance assay predicts improved survival in patients with advanced epithelial ovarian, fallopian, and peritoneal cancer. Int J Cancer. 2009 Jun 15; Epub ahead of print. - PubMed
-
- Oncotech. [as of May 6, 2009];EDR Assay®. http://www.exiqon.com/dxps/Documents/edr_4_pager.pdf.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

