Aup1-mediated regulation of Rtg3 during mitophagy
- PMID: 19840933
- PMCID: PMC2791017
- DOI: 10.1074/jbc.M109.048140
Aup1-mediated regulation of Rtg3 during mitophagy
Abstract
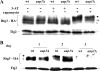
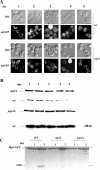
Mitophagy is an autophagic process that degrades mitochondria by an intracellular engulfment that leads to their delivery into the lumen of the cell's hydrolytic compartment, such as the lysosome in animal cells or the vacuole in yeast. It is hypothesized that such processes serve a quality control function to prevent or slow the accumulation of malfunctioning mitochondria, which are thought in turn to underlie central aspects of the aging process in eukaryotic organisms. We recently identified a conserved mitochondrial protein phosphatase homolog, Aup1, which is required for efficient stationary phase mitophagy in yeast. In the present report, we demonstrate that the retrograde signaling pathway (RTG) is defective in aup1Delta mutants. In agreement with a role for Aup1 in the regulation of the RTG pathway, we find that deletion of RTG3, a transcription factor that mediates the RTG response, causes a defect in stationary phase mitophagy and that deletion of AUP1 leads to changes in Rtg3 phosphorylation patterns under these conditions. In addition, we find that mitophagic conditions lead to induction of RTG pathway target genes in an Aup1-dependent fashion. Thus, our results suggest that the function of Aup1 in mitophagy could be explained through its regulation of Rtg3-dependent transcription.
Figures







Similar articles
-
Phosphorylation of mitochondrial matrix proteins regulates their selective mitophagic degradation.Proc Natl Acad Sci U S A. 2019 Oct 8;116(41):20517-20527. doi: 10.1073/pnas.1901759116. Epub 2019 Sep 23. Proc Natl Acad Sci U S A. 2019. PMID: 31548421 Free PMC article.
-
Aup1p, a yeast mitochondrial protein phosphatase homolog, is required for efficient stationary phase mitophagy and cell survival.J Biol Chem. 2007 Feb 23;282(8):5617-24. doi: 10.1074/jbc.M605940200. Epub 2006 Dec 13. J Biol Chem. 2007. PMID: 17166847
-
Accounting for strain-specific differences during RTG target gene regulation in Saccharomyces cerevisiae.FEMS Yeast Res. 2006 Jan;6(1):112-9. doi: 10.1111/j.1567-1364.2005.00008.x. FEMS Yeast Res. 2006. PMID: 16423076
-
Stationary-phase mitophagy in respiring Saccharomyces cerevisiae.Antioxid Redox Signal. 2011 May 15;14(10):2003-11. doi: 10.1089/ars.2010.3807. Epub 2011 Mar 8. Antioxid Redox Signal. 2011. PMID: 21194383 Review.
-
Retrograde Signaling as a Mechanism of Yeast Adaptation to Unfavorable Factors.Biochemistry (Mosc). 2018 Feb;83(2):98-106. doi: 10.1134/S0006297918020025. Biochemistry (Mosc). 2018. PMID: 29618296 Review.
Cited by
-
The pyruvate dehydrogenase complex regulates mitophagic trafficking and protein phosphorylation.Life Sci Alliance. 2023 Jul 13;6(9):e202302149. doi: 10.26508/lsa.202302149. Print 2023 Sep. Life Sci Alliance. 2023. PMID: 37442609 Free PMC article.
-
Mitophagy and Parkinson's disease: the PINK1-parkin link.Biochim Biophys Acta. 2011 Apr;1813(4):623-33. doi: 10.1016/j.bbamcr.2010.08.007. Epub 2010 Aug 21. Biochim Biophys Acta. 2011. PMID: 20736035 Free PMC article. Review.
-
Mitochondria orchestrate proteostatic and metabolic stress responses.EMBO Rep. 2019 Oct 4;20(10):e47865. doi: 10.15252/embr.201947865. Epub 2019 Sep 18. EMBO Rep. 2019. PMID: 31531937 Free PMC article. Review.
-
Ptc6 is required for proper rapamycin-induced down-regulation of the genes coding for ribosomal and rRNA processing proteins in S. cerevisiae.PLoS One. 2013 May 21;8(5):e64470. doi: 10.1371/journal.pone.0064470. Print 2013. PLoS One. 2013. PMID: 23704987 Free PMC article.
-
Yeast as a tool to study signaling pathways in mitochondrial stress response and cytoprotection.ScientificWorldJournal. 2012;2012:912147. doi: 10.1100/2012/912147. Epub 2012 Feb 2. ScientificWorldJournal. 2012. PMID: 22454613 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

