GRASP65 and GRASP55 sequentially promote the transport of C-terminal valine-bearing cargos to and through the Golgi complex
- PMID: 19840934
- PMCID: PMC2787347
- DOI: 10.1074/jbc.M109.068403
GRASP65 and GRASP55 sequentially promote the transport of C-terminal valine-bearing cargos to and through the Golgi complex
Abstract
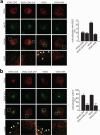
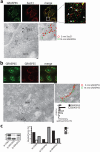
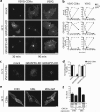
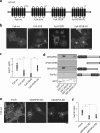
The Golgi matrix proteins GRASP65 and GRASP55 have recognized roles in maintaining the architecture of the Golgi complex, in mitotic progression and in unconventional protein secretion whereas, surprisingly, they have been shown to be dispensable for the transport of commonly used reporter cargo proteins along the secretory pathway. However, it is becoming increasingly clear that many trafficking machineries operate in a cargo-specific manner, thus we have investigated whether GRASPs may control the trafficking of selected classes of cargo. We have taken into consideration the C-terminal valine-bearing receptors CD8alpha and Frizzled4 that we show bind directly to the PSD95-DlgA-zo-1 (PDZ) domains of GRASP65 and GRASP55. We demonstrate that both GRASPs are needed sequentially for the efficient transport to and through the Golgi complex of these receptors, thus highlighting a novel role for the GRASPs in membrane trafficking. Our results open new perspectives for our understanding of the regulation of surface expression of a class of membrane proteins, and suggests the causal mechanisms of a dominant form of autosomal human familial exudative vitreoretinopathy that arises from the Frizzled4 mutation involving its C-terminal valine.
Figures



References
-
- Barr F. A., Puype M., Vandekerckhove J., Warren G. (1997) Cell 91, 253–262 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

