Genetic ablation of calcium-independent phospholipase A2{gamma} leads to alterations in hippocampal cardiolipin content and molecular species distribution, mitochondrial degeneration, autophagy, and cognitive dysfunction
- PMID: 19840936
- PMCID: PMC2790994
- DOI: 10.1074/jbc.M109.055194
Genetic ablation of calcium-independent phospholipase A2{gamma} leads to alterations in hippocampal cardiolipin content and molecular species distribution, mitochondrial degeneration, autophagy, and cognitive dysfunction
Abstract
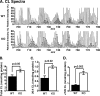
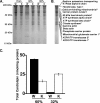
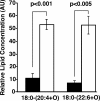
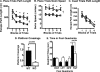
Genetic ablation of calcium-independent phospholipase A(2)gamma (iPLA(2)gamma) results in profound alterations in hippocampal phospholipid metabolism and mitochondrial phospholipid homeostasis resulting in enlarged and degenerating mitochondria leading to autophagy and cognitive dysfunction. Shotgun lipidomics demonstrated multiple alterations in hippocampal lipid metabolism in iPLA(2)gamma(-/-) mice including: 1) a markedly elevated hippocampal cardiolipin content with an altered molecular species composition characterized by a shift to shorter chain length molecular species; 2) alterations in both choline and ethanolamine glycerophospholipids, including a decreased plasmenylethanolamine content; 3) increased oxidized phosphatidylethanolamine molecular species; and 4) an increased content of ceramides. Electron microscopic examination demonstrated the presence of enlarged heteromorphic lamellar structures undergoing degeneration accompanied by the presence of ubiquitin positive spheroid inclusion bodies. Purification of these enlarged heteromorphic lamellar structures by buoyant density centrifugation and subsequent SDS-PAGE and proteomics identified them as degenerating mitochondria. Collectively, these results identify the obligatory role of iPLA(2)gamma in neuronal mitochondrial lipid metabolism and membrane structure demonstrating that iPLA(2)gamma loss of function results in a mitochondrial neurodegenerative disorder characterized by degenerating mitochondria, autophagy, and cognitive dysfunction.
Figures







References
-
- Kann O., Kovács R. (2007) Am. J. Physiol. Cell Physiol. 292, C641–C657 - PubMed
-
- Dawson T. M., Dawson V. L. (2003) Science 302, 819–822 - PubMed
-
- Lin M. T., Beal M. F. (2006) Nature 443, 787–795 - PubMed
-
- Keil U., Bonert A., Marques C. A., Scherping I., Weyermann J., Strosznajder J. B., Müller-Spahn F., Haass C., Czech C., Pradier L., Müller W. E., Eckert A. (2004) J. Biol. Chem. 279, 50310–50320 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK19645-28/DK/NIDDK NIH HHS/United States
- P30 NS057105/NS/NINDS NIH HHS/United States
- P01 HL57278/HL/NHLBI NIH HHS/United States
- R01 DK019645/DK/NIDDK NIH HHS/United States
- K08 NS048924/NS/NINDS NIH HHS/United States
- R01 HL41250/HL/NHLBI NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- P60 DK020579/DK/NIDDK NIH HHS/United States
- R37 DK019645/DK/NIDDK NIH HHS/United States
- P01 HL057278/HL/NHLBI NIH HHS/United States
- R01 AG023168/AG/NIA NIH HHS/United States
- NS48924/NS/NINDS NIH HHS/United States
- R01 HL041250/HL/NHLBI NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
- R01 AG23168/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

