N-terminal domains elicit formation of functional Pmel17 amyloid fibrils
- PMID: 19840945
- PMCID: PMC2790984
- DOI: 10.1074/jbc.M109.047449
N-terminal domains elicit formation of functional Pmel17 amyloid fibrils
Abstract
Pmel17 is a transmembrane protein that mediates the early steps in the formation of melanosomes, the subcellular organelles of melanocytes in which melanin pigments are synthesized and stored. In melanosome precursor organelles, proteolytic fragments of Pmel17 form insoluble, amyloid-like fibrils upon which melanins are deposited during melanosome maturation. The mechanism(s) by which Pmel17 becomes competent to form amyloid are not fully understood. To better understand how amyloid formation is regulated, we have defined the domains within Pmel17 that promote fibril formation in vitro. Using purified recombinant fragments of Pmel17, we show that two regions, an N-terminal domain of unknown structure and a downstream domain with homology to a polycystic kidney disease-1 repeat, efficiently form amyloid in vitro. Analyses of fibrils formed in melanocytes confirm that the polycystic kidney disease-1 domain forms at least part of the physiological amyloid core. Interestingly, this same domain is also required for the intracellular trafficking of Pmel17 to multivesicular compartments within which fibrils begin to form. Although a domain of imperfect repeats (RPT) is required for fibril formation in vivo and is a component of fibrils in melanosomes, RPT is not necessary for fibril formation in vitro and in isolation is unable to adopt an amyloid fold in a physiologically relevant time frame. These data define the structural core of Pmel17 amyloid, imply that the RPT domain plays a regulatory role in timing amyloid conversion, and suggest that fibril formation might be physically linked with multivesicular body sorting.
Figures

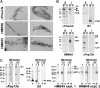


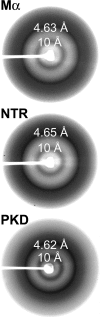


References
-
- Chakraborty A. K., Platt J. T., Kim K. K., Kwon B. S., Bennett D. C., Pawelek J. M. (1996) Eur. J. Biochem. 236, 180–188 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

