Risk of bias versus quality assessment of randomised controlled trials: cross sectional study
- PMID: 19841007
- PMCID: PMC2764034
- DOI: 10.1136/bmj.b4012
Risk of bias versus quality assessment of randomised controlled trials: cross sectional study
Abstract
Objectives: To evaluate the risk of bias tool, introduced by the Cochrane Collaboration for assessing the internal validity of randomised trials, for inter-rater agreement, concurrent validity compared with the Jadad scale and Schulz approach to allocation concealment, and the relation between risk of bias and effect estimates.
Design: Cross sectional study. Study sample 163 trials in children.
Main outcome measures: Inter-rater agreement between reviewers assessing trials using the risk of bias tool (weighted kappa), time to apply the risk of bias tool compared with other approaches to quality assessment (paired t test), degree of correlation for overall risk compared with overall quality scores (Kendall's tau statistic), and magnitude of effect estimates for studies classified as being at high, unclear, or low risk of bias (metaregression).
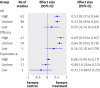
Results: Inter-rater agreement on individual domains of the risk of bias tool ranged from slight (kappa=0.13) to substantial (kappa=0.74). The mean time to complete the risk of bias tool was significantly longer than for the Jadad scale and Schulz approach, individually or combined (8.8 minutes (SD 2.2) per study v 2.0 (SD 0.8), P<0.001). There was low correlation between risk of bias overall compared with the Jadad scores (P=0.395) and Schulz approach (P=0.064). Effect sizes differed between studies assessed as being at high or unclear risk of bias (0.52) compared with those at low risk (0.23).
Conclusions: Inter-rater agreement varied across domains of the risk of bias tool. Generally, agreement was poorer for those items that required more judgment. There was low correlation between assessments of overall risk of bias and two common approaches to quality assessment: the Jadad scale and Schulz approach to allocation concealment. Overall risk of bias as assessed by the risk of bias tool differentiated effect estimates, with more conservative estimates for studies at low risk.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Agency for Healthcare Research and Quality. Systems to rate the strength of scientific evidence. Evidence report/technology assessment No 47. 2002. www.ahrq.gov/clinic/epcsums/strengthsum.htm. - PMC - PubMed
-
- Verhagen AP, de Vet HC, de Bie RA, Boers M, van den Brandt PA. The art of quality assessment of RCTs included in systematic reviews. J Clin Epidemiol 2001;54:651-4. - PubMed
-
- Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions version 5.0.0. Cochrane Collaboration, 2008.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources