Pharmacokinetics of artemether-lumefantrine and artesunate-amodiaquine in children in Kampala, Uganda
- PMID: 19841149
- PMCID: PMC2798532
- DOI: 10.1128/AAC.00679-09
Pharmacokinetics of artemether-lumefantrine and artesunate-amodiaquine in children in Kampala, Uganda
Abstract
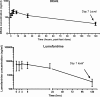
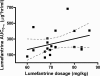
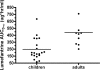
The World Health Organization recommends the use of artemisinin-based combination therapies (ACTs) for the treatment of uncomplicated malaria. The two most widely adopted ACT regimens are artemether (AR)-lumefantrine (LR) (the combination is abbreviated AL) and amodiaquine (AQ)-artesunate (AS). Pharmacokinetic (PK) data informing the optimum dosing of these drug regimens is limited, especially in children. We evaluated PK parameters in Ugandan children aged 5 to 13 years with uncomplicated malaria treated with AL (n = 20) or AQ-AS (n = 21), with intensive venous sampling occurring at 0, 2, 4, 8, 24, and 120 h following administration of the last dose of 3-day regimens of AL (twice daily) or AQ-AS (once daily). AS achieved an estimated maximum concentration in plasma (C(max)) of 51 ng/ml and an area under the concentration-time curve from time zero to infinity (AUC(0-infinity)) of 113 ng.h/ml; and its active metabolite, dihydroartemisinin (DHA), achieved a geometric mean C(max) of 473 ng/ml and an AUC(0-infinity) of 1,404 ng.h/ml. AR-DHA exhibited a C(max) of 34/119 ng/ml and an AUC(0-infinity) of 168/382 ng.h/ml, respectively. For LR, C(max) and AUC(0-infinity) were 6,757 ng/ml and 210 microg.h/ml, respectively. For AQ and its active metabolite, desethylamodiaquine (DEAQ), the C(max)s were 5.2 ng/ml and 235 ng/ml, respectively, and the AUC(0-infinity)s were 39.3 ng.h/ml and 148 microg.h/ml, respectively. Comparison of the findings of the present study to previously published data for adults suggests that the level of exposure to LR is lower in children than in adults and that the level of AQ-DEAQ exposure is similar in children and adults. For the artemisinin derivatives, differences between children and adults were variable and drug specific. The PK results generated for children must be considered to optimize the dosing strategies for these widely utilized ACT regimens.
Figures

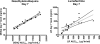
References
-
- Adjei, G. O., K. Kristensen, B. Q. Goka, L. C. Hoegberg, M. Alifrangis, O. P. Rodrigues, and J. A. Kurtzhals. 2008. Effect of concomitant artesunate administration and cytochrome P4502C8 polymorphisms on the pharmacokinetics of amodiaquine in Ghanaian children with uncomplicated malaria. Antimicrob. Agents Chemother. 52:4400-4406. - PMC - PubMed
-
- Ashley, E. A., K. Stepniewska, N. Lindegardh, R. McGready, A. Annerberg, R. Hutagalung, T. Singtoroj, G. Hla, A. Brockman, S. Proux, J. Wilahphaingern, P. Singhasivanon, N. J. White, and F. Nosten. 2007. Pharmacokinetic study of artemether-lumefantrine given once daily for the treatment of uncomplicated multidrug-resistant falciparum malaria. Trop. Med. Int. Health 12:201-208. - PubMed
-
- Aubouy, A., M. Bakary, A. Keundjian, B. Mbomat, J. R. Makita, F. Migot-Nabias, M. Cot, J. Le Bras, and P. Deloron. 2003. Combination of drug level measurement and parasite genotyping data for improved assessment of amodiaquine and sulfadoxine-pyrimethamine efficacies in treating Plasmodium falciparum malaria in Gabonese children. Antimicrob. Agents Chemother. 47:231-237. - PMC - PubMed
-
- Barnes, K. I., F. Little, P. J. Smith, A. Evans, W. M. Watkins, and N. J. White. 2006. Sulfadoxine-pyrimethamine pharmacokinetics in malaria: pediatric dosing implications. Clin. Pharmacol. Ther. 80:582-596. - PubMed
-
- Barnes, K. I., W. M. Watkins, and N. J. White. 2008. Antimalarial dosing regimens and drug resistance. Trends Parasitol. 24:127-134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

