Zyklophin, a systemically active selective kappa opioid receptor peptide antagonist with short duration of action
- PMID: 19841255
- PMCID: PMC2775281
- DOI: 10.1073/pnas.0910180106
Zyklophin, a systemically active selective kappa opioid receptor peptide antagonist with short duration of action
Abstract
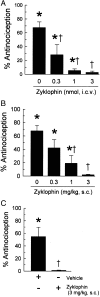
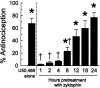
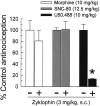
The cyclic peptide zyklophin {[N-benzylTyr(1),cyclo(D-Asp(5),Dap(8))-dynorphin A-(1-11)NH(2), Patkar KA, et al. (2005) J Med Chem 48: 4500-4503} is a selective peptide kappa opioid receptor (KOR) antagonist that shows activity following systemic administration. Systemic (1-3 mg/kg s.c.) as well as central (0.3-3 nmol intracerebroventricular, i.c.v.) administration of this peptide dose-dependently antagonizes the antinociception induced by the selective KOR agonist U50,488 in C57BL/6J mice tested in the 55 degrees C warm water tail withdrawal assay. Zyklophin administration had no effect on morphine- or SNC-80-mediated antinociception, suggesting that zyklophin selectively antagonizes KOR in vivo. Additionally, the antagonism of antinociception induced by centrally (i.c.v.) administered U50,488 following peripheral administration of zyklophin strongly suggests that the peptide crosses the blood-brain barrier to antagonize KOR in the CNS. Most importantly, the antagonist activity of zyklophin (3 mg/kg s.c.) lasts less than 12 h, which contrasts sharply with the exceptionally long duration of antagonism reported for the established small-molecule selective KOR antagonists such as nor-binaltorphimine (nor-BNI) that last weeks after a single administration. Systemically administered zyklophin (3 mg/kg s.c.) also prevented stress-induced reinstatement of cocaine-seeking behavior in a conditioned place preference assay. In conclusion, the peptide zyklophin is a KOR-selective antagonist that exhibits the desired shorter duration of action, and represents a significant advance in the development of KOR-selective antagonists.
Conflict of interest statement
Conflict of interest statement: The authors have filed a U.S. patent application on using zyklophin entitled “Method for Treating and/or Preventing Drug Seeking Behavior.”
Figures

References
-
- Binder W, Carmody J, Walker J. Effect of gender on anti-inflammatory and analgesic actions of two κ-opioids. J Pharmacol Exp Ther. 2000;292:303–309. - PubMed
-
- Mello N, Negus SS. Interactions between kappa opioid agonists and cocaine. Preclinical studies. Ann N Y Acad Sci. 2000;909:104–132. - PubMed
-
- Mague SD, et al. Antidepressant-like effects of κ-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. - PubMed
-
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology. 2005;183:118–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

