An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris
- PMID: 19841260
- PMCID: PMC2774033
- DOI: 10.1073/pnas.0905010106
An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris
Abstract
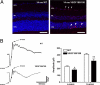
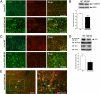
Clinical and experimental observations indicate a role for VEGF secreted by the retinal pigment epithelium (RPE) in the maintenance of the choriocapillaris (CC). VEGF in mice is produced as three isoforms, VEGF120, VEGF164, and VEGF188, that differ in their ability to bind heparan sulfate proteoglycan. RPE normally produces the more soluble isoforms, VEGF120 and VEGF164, but virtually no VEGF188, reflecting the fact that molecules secreted by the RPE must diffuse across Bruch's membrane (BrM) to reach the choriocapillaris. To determine the role of RPE-derived soluble VEGF on the choriocapillaris survival, we used mice that produce only VEGF188. VEGF188/188 mice exhibited normal choriocapillaris development. However, beginning at 7 months of age, we observed a progressive degeneration characterized by choriocapillaris atrophy, RPE and BrM abnormalities, culminating in areas of RPE loss and dramatic choroidal remodeling. Increased photoreceptor apoptosis in aged VEGF188/188 mice led to a decline in visual acuity as detected by electroretinogram (ERG). These changes are reminiscent of geographic atrophy (GA) and point to a role for RPE-derived VEGF in the maintenance of the choriocapillaris.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


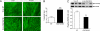
References
-
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. - PubMed
-
- Soker S, Miao HQ, Nomi M, Takashima S, Klagsbrun M. VEGF165 mediates formation of complexes containing VEGFR-2 and neuropilin-1 that enhance VEGF165-receptor binding. J Cell Biochem. 2002;85:357–368. - PubMed
-
- Eremina V, Quaggin SE. The role of VEGF-A in glomerular development and function. Curr Opin Nephrol Hypertens. 2004;13:9–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

