Targeting proteins for degradation
- PMID: 19841631
- PMCID: PMC4228941
- DOI: 10.1038/nchembio.250
Targeting proteins for degradation
Erratum in
- Nat Chem Biol. 2009 Dec;5(12):954
Abstract
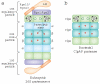
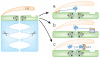
Protein degradation plays a central role in many cellular functions. Misfolded and damaged proteins are removed from the cell to avoid toxicity. The concentrations of regulatory proteins are adjusted by degradation at the appropriate time. Both foreign and native proteins are digested into small peptides as part of the adaptive immune response. In eukaryotic cells, an ATP-dependent protease called the proteasome is responsible for much of this proteolysis. Proteins are targeted for proteasomal degradation by a two-part degron, which consists of a proteasome binding signal and a degradation initiation site. Here we describe how both components contribute to the specificity of degradation.
Figures
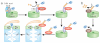

References
-
- Lee C, Schwartz MP, Prakash S, Iwakura M, Matouschek A. ATP-Dependent Proteases Degrade Their Substrates by Processively Unraveling Them from the Degradation Signal. Mol Cell. 2001;7:627–637. - PubMed
-
- Groll M, et al. Structure of 20S proteasome from yeast at 2.4 Å resolution. Nature. 1997;386:463–471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

