Recent synthetic studies leading to structural revisions of marine natural products
- PMID: 19841716
- PMCID: PMC2763102
- DOI: 10.3390/md7030314
Recent synthetic studies leading to structural revisions of marine natural products
Abstract
Because of the highly unique structures of marine natural products, there are many examples of structures that were originally proposed based on spectral analyses but later proven incorrect. In many cases, the total syntheses of the originally proposed structures of marine natural products has confirmed their incorrectness and the subsequent total syntheses of the newly proposed structures proved the revised structures. This review will show such cases appearing after 2005 and demonstrate how the true structures were elucidated.
Keywords: marine natural product; stereoselective; structural revision; synthesis.
Figures

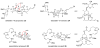
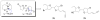
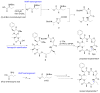
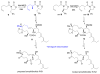
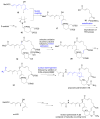


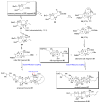





References
-
- Newman DJ, Cragg GM. Natural products from marine invertebrates and microbes as modulators of antitumor targets. Curr Drug Targets. 2006;7:279–304. - PubMed
-
- Newman DJ, Cragg GM. Advanced preclinical and clinical trials of natural products and related compounds from marine sources. Curr Med Chem. 2004;11:1693–1713. - PubMed
-
- Butler MS. Natural products to drugs: natural product derived compounds in clinical trials. Nat Prod Rep. 2005;22:162–195. - PubMed
-
- Usami Y, Ueda Y. Synthetic study toward antitumour natural product pericosine A. Chem Lett. 2005;34:1062–1063.
-
- Usami Y, Ueda Y. Stereoselective syntheses of diastereomers of antitumor natural product pericosine a from (−)-quinic acid. Synthesis. 2007:3219–3225.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
